AbbVie 2017: Humira, And What’s Next
While the world’s leading prescription drug still accounts for the majority of AbbVie’s revenue, plenty else is bubbling up at the almost-five-year-old company.
AbbVie Inc.
1 North Waukegan Road
North Chicago, IL 60064
Telephone: 847-932-7900
Website: abbvie.com
Best-Selling Products
| Product | 2016 Sales | 2015 Sales |
|---|---|---|
| Humira |
$16,078 |
$14,012 |
|
Imbruvica |
$1,832 | $754 |
| Viekira | $1,522 | $1,639 |
| Lupron | $821 | $826 |
| Synthroid | $763 |
$755 |
| Creon | $730 |
$632 |
| Synagis | $730 | $740 |
| AndroGel | $675 | $694 |
| Kaletra | $549 | $700 |
All sales are in millions of dollars.
Financial Performance
| 2016 | 2015 | |
|---|---|---|
| Revenue | $25,638 | $22,859 |
| Net income | $5,953 | $5,144 |
| Diluted EPS | $3.63 | $3.13 |
| R&D expense | $4,366 | $4,285 |
| 1H2017 | 1H2016 | |
|---|---|---|
| Revenue |
$13,482 |
$12,410 |
| Net income | $3,626 | $2,964 |
| Diluted EPS | $2.25 | $1.81 |
| R&D expense | $2,358 | $2,070 |
In millions of dollars, except EPS
While not quite as impressive as the previous annum’s Company of the Year performance, 2016 was very good for AbbVie. Humira, of course, remained the No. 1 prescription product by sales in the world for the fifth consecutive year by a considerable margin, with sales clearing $16 billion for the first time. And while biosimilar competition for Humira may be waiting in the wings, a new blockbuster, the oncology drug Imbruvica, added more than a billion dollars to its sales in 2016, with two more FDA approvals for the product arriving in 2017 so far. And plenty of good things are brewing in the pipeline, with positive results coming in recently for the first-in-class oncology drug Venclyxto and the autoimmune drug upadacitinib, not to mention FDA’s approval of Mavyret for hepatitis C. All in all, not too bad for a company that’s still just four years old.
“By nearly any measure, our results over our first four years place us solidly in the top tier of our peer group,” says Richard Gonzalez, chairman and CEO of AbbVie. “Since inception, we’ve delivered compounded annual revenue and adjusted earnings per share growth of 11 percent and 15 percent, respectively. Over the same period, our dividend has grown 60 percent, contributing to a total shareholder return of 111 percent, the highest among our peer group. In addition, we’ve launched more than a dozen new products or expanded indications and built one of the strongest pipelines in the industry with the potential for leadership across many therapeutic areas.”

“By nearly any measure, our results over our first four years place us solidly in the top tier of our peer group,” says Richard Gonzalez, chairman and CEO of AbbVie.
AbbVie’s revenue in 2016 totaled $25.64 billion, an improvement of 12.2 percent over the previous year. Net income rose by 15.7 percent to $5.95 billion and earnings per share were up 50 cents to $3.63. In the first half of 2017, the company’s revenue rose 8.6 percent to $13.48 billion, with net income up 22.3 percent to $3.63 billion and EPS rising 44 cents to $2.25. Company executives are projecting full-year 2017 EPS at between $4.55 and $4.65.
Product Performance
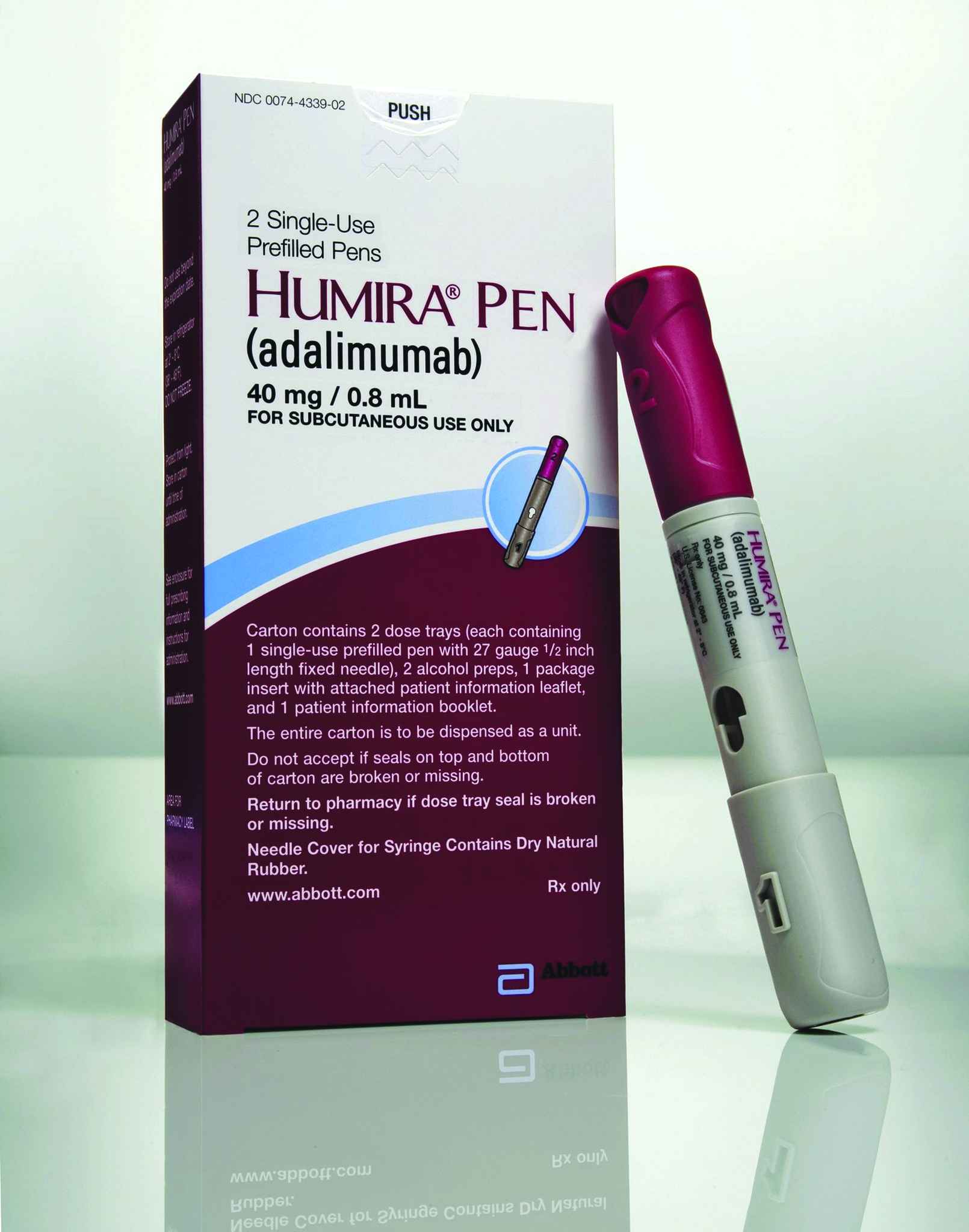
Still atop AbbVie’s sales charts, and everyone else’s too, Humira generated $16.08 billion in sales for the company in 2016, an improvement of 14.7 percent compared with the previous year – not to mention $7.4 billion more than the world’s second-leading prescription drug, Harvoni. In the first half of 2017, Humira sales rose another 14.3 percent to $8.83 billion. While FDA has approved two biosimilars to Humira so far – Amgen’s Amjevita in September 2016 and Boehringer Ingelheim’s Cyltezo in August 2017 – published reports suggest that these will not be launched until at least 2018, if then, due to ongoing patent litigation. Humira is presently approved for the treatment of 10 autoimmune diseases in the United States and 12 in the EU.
In March, FDA approved the inclusion of moderate to severe fingernail psoriasis data in the Humira prescribing information for patients with moderate to severe chronic plaque psoriasis. This approval made Humira the first biologic treatment with data on fingernail psoriasis in its U.S. prescribing information. Fingernail psoriasis, which affects half of all psoriasis patients, is a form of chronic plaque psoriasis characterized by pitting, deformation, thickening, discoloration, pain, and separation of the nail from the nail bed. The study on which the new approval was based demonstrated that nearly half of adult patients treated with Humira achieved an assessment of clear or minimal with at least a two-grade improvement from baseline in signs and symptoms of fingernail psoriasis compared to 6.9 percent of placebo patients.
In June, AbbVie presented results from a 78-week post-marketing observational study showing that participation in an AbbVie patient support program in the EU, Switzerland, Israel, Mexico, Puerto Rico, and Australia was associated with statistically significant improvement in functional outcomes in patients with moderate to severe rheumatoid arthritis who initiated Humira therapy. The primary endpoint of the PASSION study was the percentage of study participants who achieved a minimal clinically important difference (MCID, or improvement of ≥ 0.22) in the Health Assessment Questionnaire Disability Index (HAQ-DI), a patient-reported questionnaire to measure RA function, at week 78, compared to baseline. Results showed that 72.1 percent of patients achieved the MCID in HAQ-DI at week 78 versus baseline (as observed). PASSION also evaluated the potential impact of patient support program participation on functional outcomes in patients with moderate to severe RA compared to patients not participating in a support program. Results demonstrated that 48.1 percent of PSP participants achieved HAQ-DI MCID, compared to 37.8 percent of nonparticipants, at week 78, utilizing NRI.

Humira retained its title as the top-selling prescription drug in the world in 2016, by a margin of more than $7 billion.
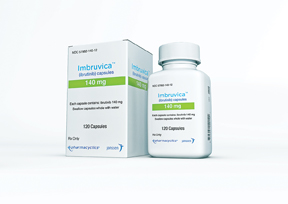
Sales of Imbruvica, AbbVie’s leading oncology product, more than doubled in 2016, growing from $754 million to $1.83 billion. According to company executives, this was primarily due to last year’s FDA and EMA approvals for the first-line treatment of patients with chronic lymphocytic leukemia. In the first half of 2017, Imbruvica sales were up another 43.5 percent to $1.18 billion. Imbruvica was acquired in the Pharmacyclics transaction in May 2015.
In January, FDA approved Imbruvica for the treatment of patients with relapsed/refractory marginal zone lymphoma (MZL) who require systemic therapy and have received at least one prior anti-CD20-based therapy. This indication was approved under accelerated approval based on overall response rate, and continued approval may be contingent upon verification and description of clinical benefit in a confirmatory trial.
The marketing approval for the MZL indication was based on data from the Phase II, open-label, multi-center, single-arm PCYC-1121 study, which evaluated the safety and efficacy of Imbruvica in MZL patients who require systemic therapy and have received at least one prior anti-CD20-based therapy. The efficacy analysis included 63 patients with three sub-types of MZL: mucosa-associated lymphoid tissue, nodal, and splenic. The ORR was achieved in nearly half (46 percent) of the patients as assessed by an Independent Review Committee using criteria adopted from the International Working Group criteria for malignant lymphoma, with efficacy observed across all three MZL sub-types. In the clinical trial, 3.2 percent of patients had a complete response and 42.9 percent of patients had a partial response.
In August, FDA approved Imbruvica for the treatment of adult patients with chronic graft-versus-host-disease (cGVHD) after failure of one or more lines of systemic therapy. With this approval, Imbruvica became the first therapy specifically approved for adults with cGVHD, a serious and debilitating potential consequence of stem cell or bone marrow transplant.
The approval in cGVHD was based on findings from an open-label, multi-center, single-arm Phase Ib/II trial (PCYC-1129), which evaluated the safety and efficacy of Imbruvica in 42 patients with cGVHD that failed to respond to first-line corticosteroid therapy and required additional therapy. The most common underlying malignancies leading to transplantation for the study population were acute lymphocytic leukemia, acute myeloid leukemia, and chronic lymphocytic leukemia. The median time since cGVHD diagnosis was 14 months, and the median number of prior cGVHD treatments was two. The majority of patients (88 percent) had at least two organs affected at baseline, including mouth (86 percent), skin (81 percent) and gastrointestinal tract (33 percent).
“FDA’s approval of Imbruvica in chronic graft-versus-host-disease after failure of one or more lines of systemic therapy addresses an area of high unmet medical need for patients and marks the first approved use for the therapy outside of blood cancers,” says Lori Styles, M.D., Senior Medical Director and GVHD program clinical lead at Pharmacyclics LLC, an AbbVie company. “This approval is an indicator of what is possible with Imbruvica, and we remain excited about the clinical utility of Imbruvica in other disease areas. We continue to explore the full potential of this therapy and believe our comprehensive clinical trial program will help advance patient care.”
Sales of the hepatitis C product Viekira fell 7.1 percent in 2016, to $1.52 billion. According to AbbVie executives, this was due to lower market share – primarily in the United States – market contraction, and price erosion. In the first half of 2017, Viekira sales nearly halved, from $833 million to $488 million.
Creon, AbbVie’s market-leading pancreatic enzyme product, enjoyed sales growth of 15.5 percent to $730 million in 2016. According to company leaders, this was due to continued market growth and higher market share. In the first half of 2017, sales of Creon rose another 15.6 percent to $381 million.
Acquisitions & Collaborations
During January, AbbVie simultaneously announced a series of four new collaborations. First, the company and Pure MHC, a privately held target discovery company, signed a research and license agreement to discover and validate peptide targets for use with T-cell receptor therapeutics in several types of cancers. T-cells play a key role in regulating immune responses to tumors. Receptors on the surface of T-cells recognize tumor antigens in the form of small peptides and provide a selective pathway for targeted cancer therapies. Pure MHC has developed technology to identify novel, tumor-associated peptides based on innovation licensed from the University of Oklahoma. The collaboration between AbbVie and Pure MHC will seek to identify a library of peptide targets for further research across multiple tumor types and advance AbbVie’s ongoing development of next-generation immuno-oncology therapies.
Second, AbbVie entered into an exclusive license with Dong-A-ST, a specialty healthcare company in South Korea, for MerTK inhibitors in pre-clinical development for use in conjunction with immuno-oncology therapies. MerTK is a protein that is believed to contribute to the promotion of immunosuppressive tumor microenvironment. Inhibition of this activity may help promote an inflammatory state, alerting the immune system to attack tumors and augmenting the efficacy of targeted cancer therapies such as checkpoint inhibitors (anti-PD1/PD-L1) and pro-apoptotic agents. The collaboration will explore the combination of MerTK inhibitors in conjunction with AbbVie’s portfolio of anticancer agents across multiple types of solid tumors. The addition of this mechanism, company leaders say, will advance AbbVie’s existing research into immuno-oncology therapies and complement its oncology pipeline under development for nearly 20 cancers and tumor types.
Third, AbbVie and Zebra Biologics Inc., a discovery-stage biotechnology company, entered into a partnership to discover agonist antibody therapeutics for inflammatory diseases. Zebra will utilize its novel and patented function-based antibody discovery platform to generate antibodies that activate biological pathways associated with targets designated by AbbVie. Zebra and AbbVie will collaborate closely on the identification and pre-clinical validation of emerging candidates. The targets were not disclosed. Zebra will lead the discovery of candidate agonist antibodies for designated targets and will collaborate with AbbVie in pre-clinical validation of select clinical candidates. Upon advancement of clinical candidates, AbbVie would be responsible for clinical development, manufacturing, regulatory approval and world-wide commercialization.
Fourth, AbbVie and Genomics Medicine Ireland, a life-sciences startup company, partnered to conduct population genomics research in Ireland. The alliance will sequence the genomes of 45,000 volunteers across Ireland. The data to be included will originate from people with several types of immune-mediated diseases, neurological disorders, and cancer, as well as people unaffected by these diseases. By incorporating the genotypic and phenotypic data across a wide sample population, the partnership aims to better understand human biology and disease etiology to discover new therapeutic targets and identify biomarkers. AbbVie will utilize the research to select targets for drug development, as well as potential development of companion diagnostics for selected conditions.
In March, AbbVie and M2Gen, a health informatics solutions company, announced that AbbVie had joined the Oncology Research Information Exchange Network (ORIEN) Avatar Research Program. Launched in April of 2016, the ORIEN Avatar Research Program fosters collaboration among key stakeholders in cancer research with the shared goal of discovering and developing novel therapies in clinical trials.
ORIEN Avatar is a collaboration between the 15 leading U.S. cancer hospitals that comprise ORIEN, leading pharma companies, and M2Gen, which manages the program. AbbVie is the fourth pharma company to participate in the Avatar Program.
The ORIEN Avatar Research Program was born out of the ORIEN Cancer Initiative, which launched in 2014. A first-of-its-kind effort in cancer research, ORIEN joins leading cancer centers across the United States to better study the disease and encourage collaboration in research and development across nearly 20 types of cancer. Patients at participating ORIEN hospitals donate clinical and molecular data through their consent to the Total Cancer Care Protocol, building a wealth of data that includes more than 150,000 patients to date. Cancer researchers contribute samples and disease information from patients who have consented to the Total Cancer Care Protocol. This provides rich molecular data that helps identify eligible participants for clinical trials. Pharmaceutical companies contribute financial support, and both companies and ORIEN member institutions receive access to de-identified genetic and disease information that can be used to inform the discovery and clinical development of novel cancer therapeutics. By matching the right patient to the right trial, ORIEN Avatar seeks to accelerate the discovery and development of novel therapies for millions of patients.
“The ORIEN Avatar Research Program offers a unique and important opportunity in the effort to develop new treatments and cures for cancer,” says Steve Davidsen, VP, Oncology Discovery, AbbVie. “We see enormous potential to improve patient recruitment and targeting for clinical trials, especially more in specific subsets of patients. Through the collaborative efforts of the ORIEN Avatar participants, we can magnify the collective data on specific tumor types and biomarkers to catalyze future discoveries for patients in need.”
In June, AbbVie and Principia Biopharma Inc., a private, clinical-stage biopharmaceutical company, entered a collaboration for the development of oral immunoproteasome inhibitors. The collaboration is aimed at developing first-in-class oral therapies that bring the power of proteasome inhibition safely into the field of immunology. The collaboration contemplates the creation and development of orally bioavailable, selective inhibitors of the immunoproteasome subunits to target autoimmunity. AbbVie and Principia will collaborate on research and pre-clinical studies. Upon successful completion, AbbVie is responsible for ongoing clinical development and commercialization of viable compounds resulting from the partnership. Financial terms were not disclosed.
The immunoproteasome is a distinct class of the proteasome, abundant in immune cells. The immunoproteasome comprises multiple proteolytic subunits with specialized roles in the processing of proteins for immune system recognition. The immunoproteasome plays a unique role in immune responses, with effects on antigen presentation, T cell function and cytokine production. Selective immunoproteasome subunits can be selectively inhibited, showing therapeutic activity in preclinical autoimmune and inflammatory studies.
“Principia has discovered a novel technology that has the potential to enhance the treatment landscape for a variety of immunological diseases,” says Lisa Olson, VP, immunology research, AbbVie. “The combination of AbbVie’s world-class expertise in immunology drug development with Principia’s innovative approach to immunoproteasome inhibitors may offer patients a new class of therapies to treat serious and chronic conditions.”
In the Pipeline
In January, AbbVie announced the start of two Phase II clinical trial programs to evaluate ABBV-8E12, an investigational anti-tau antibody, in patients with early Alzheimer’s disease and progressive supranuclear palsy. In recognition of the lack of treatment options available to patients with PSP, FDA granted Fast Track Designation to ABBV-8E12. FDA and European Medicines Agency also granted Orphan Drug Designations to ABBV-8E12 for PSP.
“We see potential in ABBV-8E12 and tau-focused approaches to progressive neurodegenerative diseases, such as early Alzheimer’s disease and PSP,” says Eric Karran, VP, Foundational Neuroscience Center, AbbVie. “The initiation of the Phase II clinical trial programs and the FDA’s Fast Track Designation for PSP signify important steps forward in AbbVie’s ongoing commitment to investigating innovative scientific approaches with the hope of bringing new treatment options to patients.”
After consultation with FDA and EMA, AbbVie is beginning the Phase II clinical trial programs following completion of pre-clinical studies and a Phase I study in patients with PSP, which supported the further development of ABBV-8E12 in PSP and early Alzheimer’s disease. Positive results from the Phase I study in PSP were released at the Clinical Trials on Alzheimer’s Disease annual meeting in December 2016.
The Phase II study in early Alzheimer’s disease will enroll 400 patients to assess the efficacy and safety of ABBV-8E12 to delay disease progression. The Phase II study in PSP will evaluate 180 adults and assess the efficacy and safety of ABBV-8E12 to slow disease progression. ABBV-8E12, licensed from C2N Diagnostics in 2015, is a humanized antibody being studied to target the tau protein, which is thought to stabilize intracellular structures required for maintenance and transport in neurons. Abnormal accumulation of altered tau protein is a hallmark in a variety of neurodegenerative conditions, where the development of tau pathology strongly correlates with clinical disease progression.
In April, AbbVie announced that high SVR12 rates were achieved with 8 weeks of treatment with the company’s investigational, once-daily, ribavirin-free, pan-genotypic regimen of glecaprevir/pibrentasvir in patients with challenging to treat genotype 3 chronic hepatitis C virus infection. In results from the Phase III ENDURANCE-3 study, 95 percent (n=149/157) of GT3 chronic HCV infected patients without cirrhosis and who are new to treatment achieved sustained virologic response at 12 weeks post-treatment following 8 weeks of treatment with G/P.
As well as evaluating 8 weeks of treatment with G/P, the ENDURANCE-3 study was designed to evaluate whether 12 weeks of G/P is non-inferior to 12 weeks of sofosbuvir plus daclatasvir (SOF+DCV), a current standard of care for GT3 chronic HCV infected patients. SVR12 rates of 95 percent were seen in both 8 weeks (n=149/157) and 12 weeks (n=222/233) of treatment with G/P. Additionally, 12 weeks of treatment with G/P was demonstrated to be non-inferior to 12 weeks of treatment with SOF+DCV (97 percent, n=111/115).
GT3 is the second most common genotype globally, accounting for 18 percent of patients worldwide and 26 percent of patients in Europe. Patients with GT3 HCV have more rapid disease progression, with the highest rates of associated fibrosis, steatosis, and hepatocellular carcinoma.
Additionally during April, AbbVie announced that 99 percent (n=145/146) of chronic hepatitis C virus infected patients with genotype 1, 2, 4, 5, or 6 and compensated cirrhosis (Child-Pugh A) achieved sustained virologic response at 12 weeks post-treatment with G/P. These high SVR12 rates were seen following 12 weeks of G/P treatment without ribavirin. Patients with specific virus strains associated with resistance or with a high quantity of the virus in their bloodstream before treatment initiation were not excluded from the study.
“With our G/P clinical development program, our goal is to provide a cure for as many patients living with HCV as possible, across all genotypes and regardless of whether their disease has progressed to compensated cirrhosis,” said Michael Severino, M.D., executive VP, research and development and chief scientific officer, AbbVie. “The EXPEDITION-1 study results, along with a number of other ILC presentations from our G/P clinical development program, explore the potential of our regimen in patients with specific treatment challenges.”
In August, FDA approved G/P under the brand name Mavyret for adults with chronic hepatitis C virus infection across all major genotypes. The approval of Mavyret was supported by data from nine registrational studies in AbbVie’s clinical development program, which evaluated more than 2,300 patients in 27 countries across all major HCV genotypes and special populations. The regimen was also approved by the European Commission, under the brand name Maviret, in July.
In April, AbbVie announced that two Phase III studies evaluating veliparib, an investigational, oral poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor, did not meet their primary endpoints. The studies evaluated veliparib in combination with the chemotherapy regimen carboplatin and paclitaxel in patients with squamous non-small cell lung cancer and triple negative breast cancer.
In June, AbbVie announced the presentation of results from the pivotal Phase II study of Venclexta/Venclyxto, a first-in-class oral B-cell lymphoma-2 (BCL-2) inhibitor. Venclexta monotherapy responses in 158 patients with relapsed/refractory chronic lymphocytic leukemia and 17p deletion showed that 77 percent achieved an overall response rate (ORR = complete remission [CR] + complete remission with incomplete marrow recovery [Cri] + partial remission [PR] + nodular partial remission [nPR]), 18 percent achieved a complete remission (CR +CRi), 53 percent achieved a partial remission, 6 percent achieved an nPR, and 27 percent achieved blood minimal residual disease negativity, as measured by flow cytometry.
Venclexta monotherapy has been granted accelerated approval by FDA for use in those with CLL who have 17p deletion (deletion located on the chromosome 17 short arm) and who have been treated with at least one prior therapy; and conditional marketing authorization in the EU for the treatment of CLL in the presence of 17p deletion or TP53 mutation in adult patients who are unsuitable for or have failed a B-cell receptor pathway inhibitor, and for the treatment of CLL in the absence of 17p deletion or TP53 mutation in adult patients who have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor. The compound is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the United States and by AbbVie outside of the United States.
Also in June, AbbVie announced positive top-line results from the Phase III SELECT-NEXT clinical trial evaluating upadacitinib, an investigational oral JAK1-selective inhibitor, in patients with moderate to severe rheumatoid arthritis who did not adequately respond to treatment with conventional synthetic DMARDs. Results showed that after 12 weeks of treatment, both doses of upadacitinib (15 milligrams and 30 milligrams) met the study’s primary endpoints of ACR20 and low disease activity. Key secondary endpoints were also achieved and included ACR50, ACR70, and clinical remission.
“We are excited by these promising results for upadacitinib,” says Michael Severino, M.D., executive VP, research and development and chief scientific officer, AbbVie. “Selective inhibition of the JAK1 pathway may offer a novel treatment for rheumatoid arthritis patients who do not adequately respond to conventional therapies. We are especially encouraged by the results on the more stringent measures of efficacy, such as ACR70, low disease activity, and clinical remission. We look forward to seeing the full results from our Phase III program. AbbVie’s longstanding leadership in the treatment of immune-mediated diseases provides an opportunity to build upon our understanding and develop innovative therapies to address unmet patient needs.”
In September, AbbVie announced positive top-line results from the Phase III SELECT-BEYOND clinical trial evaluating upadacitinib in patients with moderate to severe rheumatoid arthritis who did not adequately respond or were intolerant to treatment with biologic DMARDs. Results showed that after 12 weeks of treatment, both once-daily doses of upadacitinib (15 milligrams and 30 milligrams) met the study’s primary endpoints of ACR20 and low disease activity. All ranked secondary endpoints were also achieved with both doses. The compound also showed positive results in a Phase II study in Crohn’s Disease reported in May and a Phase IIb study in atopic dermatitis reported in September.
Also in September, AbbVie and partner developer Neurocrine Biosciences Inc. submitted an NDA to FDA for elagolix, an investigational, orally administered gonadotropin-releasing hormone (GnRH) antagonist, being evaluated for the management of endometriosis with associated pain. In two replicate Phase III clinical studies, elagolix demonstrated superiority compared to placebo in reducing three types of endometriosis-associated pain – daily menstrual pelvic pain, non-menstrual pelvic pain and painful intercourse.
“The submission represents an important step forward for women suffering from endometriosis and physicians who are in need of additional medical treatment options to help manage this chronic and painful disease,” Dr. Severino says. “Elagolix has the potential to be an important oral treatment option for women suffering from the most prevalent symptoms of endometriosis and we look forward to working with the FDA throughout the review process.”
The NDA is supported by data from the largest prospective randomized endometriosis clinical trials conducted to date, which evaluated the safety and efficacy of elagolix in nearly 1,700 women with moderate-to-severe endometriosis-associated pain. The data from two replicate Phase III studies demonstrated that, at month three and month six, both elagolix doses (150 milligrams once daily and 200 milligrams twice daily) resulted in a statistically significant higher proportion of responders for menstrual pain (dysmenorrhea) and non-menstrual pelvic pain associated with endometriosis as measured by the Daily Endometriosis Pain Impact scale versus placebo. Significant improvements compared to placebo were also observed at month three for the 200 milligrams twice daily dose in scores for painful intercourse (dyspareunia). A reduction in the amount and frequency of rescue pain medication use, including nonsteroidal anti-inflammatory drugs and opioids, compared to placebo was also seen in the higher dose at month three and six. In clinical studies, elagolix treatment decreased endometrial proliferation in a dose-dependent manner after six months of treatment with no adverse endometrial findings.