After allegations of bullying became public, Dr. Eric Lander – the director of the federal government’s Office of Science and Technology Policy – resigned from his executive branch role.
The Janssen Pharmaceutical (a Johnson & Johnson company) medicine Invokana (canagliflozin) has a list of safety warnings, including ketoacidosis, a severe complication of diabetes that is potentially life-threatening. According to an investigation conducted by Reuters, J&J knew of the risk of ketoacidosis, but covered it up.
World Health Organization investigators have attempted to return to the Wuhan lab but are now being blocked by the Chinese government.
The cancer drugmaker pled guilty of violating the Federal Food, Drug and Cosmetic Act by failing to provide certain records to investigators in federal court.
A U.S. judge granted a Reuters request to unseal Merck & Co. documents produced in lawsuits related to the company’s anti-baldness treatment Propecia, finding that the public’s right to access outweighed the drug maker’s arguments for keeping the information secret.
Moderna Inc. received a report from California’s health department that several people at a center in San Diego were treated for possible allergic reactions to the company’s Covid-19 vaccine from a particular batch.
New York State Health officials are investigating a Brooklyn-based healthcare provider on suspicion it violated state guidelines for distribution of Covid-19 vaccine.
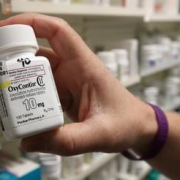
OxyContin maker Purdue Pharma pleads guilty to criminal charges
Addictions, Bankruptcy, Business, Criminal Charges, District Judges, Doctors, Former Blockbusters, Investigations, Kickbacks, New Jersey, OxyContin, Painkillers, Plea Deals, Purdue Pharma, Therapeutics, U.S. Justice Department, U.S. Opioid CrisisPurdue Pharma LP pleaded guilty to criminal charges over the handling of the company’s addictive prescription painkiller OxyContin, capping a deal with federal prosecutors to resolve an investigation into the drugmaker’s role in the U.S. opioid crisis.
A Paris-based prosecutor initiated an investigation into Sanofi over the company’s drug Depakine, which is on the market for epilepsy and bipolar disorder.
Two former executives of biotech company MiMedx Group Inc. were criminally charged with fraudulently inflating the company’s revenue.