Health officials are considering extending the eligibility for a second COVID-19 vaccine booster dose to people under 50 amid a steady rise in cases, with the United States seeing a threefold increase over the past month.
COVID is rising in the Americas, virus ‘not going away anytime soon’ – PAHO
Brazil, Central America, COVID-19 cases, COVID-19 Deaths, COVID-19 Infections, COVID-19 protocols, COVID-19 vaccination rates, Mask-wearing, North America, Pan-American Health Organization (PAHO), Social Distancing, World Health OrganizationCOVID-19 is on the rise again in the Americas as many countries have abandoned measures like masking and social distancing and many lag in vaccination rates, the Pan American Health Organization (PAHO) said on May 18.
Greece’s Health Minister Thanos Plevris announced plans to sue Novartis over what he alleges are illegal practices. The scandal has been playing out for several years, with Novartis alleged to have paid Greek public officials and health care providers to increase prescriptions for the company’s drugs and maintain higher prices.
Smoking rates declined globally for the first time on record, according to a new report on tobacco use. However, the figures from the Tobacco Atlas report also mask growing numbers of smokers in parts of the world, as well as increased tobacco use among young teenagers in almost half of the countries surveyed.
The world is no better prepared for a new pandemic than it was when coronavirus emerged in 2019 and may actually be in a worse place given the economic toll, according to a panel set up by the World Health Organization (WHO) to evaluate the global response.
U.S. House Democrats on May 17 unveiled a bill to provide $28 million in emergency funds to the Food and Drug Administration to help the regulatory agency respond to a nationwide shortage of infant formula and strengthen supervision of the industry.
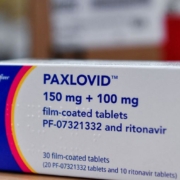
As U.S. COVID-19 cases rise, so does demand for antivirals
Antivirals, Apple, BNT162b2 (Pfizer and BioNTech), CDC, Connecticut, COVID-19 booster shots, COVID-19 cases, COVID-19 Therapeutics, COVID-19 Vaccines, Delaware, Department of Health and Human Services (HHS), Emergency Use Authorization (EUA), FDA, Health Officials, Hospitalized COVID-19 Patients, Maine, Massachusetts, Merck, Molnupiravir, New York, Northeast, Omicron (B.1.1.529) (South Africa), Omicron BA.2, Paxlovid, Pfizer, Regions, Reuters Tally, Rhode Island, Therapeutics, U.S. government, United States, White HouseRising COVID-19 cases are driving up the use of therapeutics, with Pfizer Inc.’s oral antiviral treatment Paxlovid seeing a 315 percent jump over the past four weeks, U.S. health officials said on May 17.
U.S. House Democrats on May 17 unveiled a bill to provide $28 million to the Food and Drug Administration to help respond to a nationwide shortage of infant formula.
The United States will allow baby formula imports from foreign makers that do not usually sell their products here, the Food and Drug Administration said on May 16, as it tries to ease a nationwide shortage that has left parents scrambling to feed their babies.
The U.S. Food and Drug Administration decided not to authorize the antidepressant fluvoxamine to treat COVID-19, saying the data has not shown the drug to be an effective therapeutic for fighting the virus.





 Reuters
Reuters