Short- and long-term use cases recommended by experts.
Viz.ai continues to demonstrate significant real-world evidence on the patient and economic outcomes of AI-powered care coordination; Neurolief’s neuromodulation device Relivion is approved by the Japanese Ministry of Health, Labor and Welfare; and Kanerika partners with Microsoft to build an enterprise business intelligence platform powered by Microsoft Fabric for a leading global pharmaceutical company in the United States.
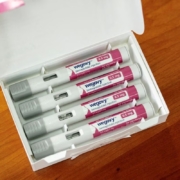
A small but rapidly growing number of U.S. adolescents began treatment with Novo Nordisk’s weight-loss drug Wegovy last year, a powerful new tool to address record rates of pediatric obesity, according to data shared exclusively with Reuters.
Pfizer has agreed to pay $93 million to settle antitrust claims by wholesale drug distributors that accused it of conspiring with India’s Ranbaxy Laboratories to delay sales of less expensive, generic versions of the cholesterol drug Lipitor.
Despite launching with just days left in 2023, Sage Therapeutics’ postpartum depression therapy Zurzuvae made $1.6 million in sales, the Massachusetts-based biotech reported in Wednesday’ s fourth-quarter and full-year earnings results.
BioSpace
While Otsuka Pharmaceutical can expect to a drop in sales as its schizophrenia therapy Abilify Maintena (aripiprazole) loses its market exclusivity later this year, the recently approved longer-acting formulation Abilify Asimtufii will help the company buttress its revenues, according to a report Wednesday from data analytics and consulting firm GlobalData.
Alvotech said on Thursday it has reached settlement agreements with Johnson & Johnson for launching a biosimilar of the pharmaceutical giant’s blockbuster psoriasis drug Stelara in Japan, Canada, and Europe this year.
With drug shortages in the U.S. not greatly improving, the Federal Trade Commission and the Department of Health and Human Services announced Wednesday that they are issuing a request for information to look at different groups involved in generic drug supply chains and evaluate how they may be contributing to persistent shortages.
Eli Lilly’s Mounjaro is being launched in Britain this week, two pharmacy companies said on Wednesday, making the UK the fourth European country to introduce the highly anticipated obesity drug.
The U.S. Food and Drug Administration on Wednesday approved Johnson & Johnson unit Actelion Pharmaceuticals’ injection, making it the first-ever treatment to treat severe frostbite in adults.








 Reuters
Reuters