Boehringer Ingelheim 2019: Solid performance
Family-owned Boehringer Ingelheim reported a satisfactory 2018 and has made some strategic acquisitions in 2019.
Boehringer Ingelheim GmbH
Binger Strasse 173
55216 Ingelheim am Rhein, Germany
Telephone: +49 6132 77 0
Website: boehringer-ingelheim.com
Best-Selling Rx Products
| Product | 2018 Sales | 2017 Sales |
|---|---|---|
| Spiriva | $2,850 | $3,339 |
| Pradaxa | $1,756 | $1,699 |
| Jardiance | $1,726 | $1,191 |
| Trajenta/Jentadueto | $1,651 | $1,575 |
| Ofev | $1,300 | $1,087 |
All sales are in millions of dollars and were translated
using the Federal Reserve Board’s average rate of
exchange in 2018: EUR 1.1817 = $1.00
Financial Performance
| 2018 | 2017 | |
|---|---|---|
| Revenue | $20,677 | $21,337 |
| Net income | $2,452 | -$264 |
| R&D expense | $3,739 | $3,637 |
| 1H 2019 | 1H 2018 | |
|---|---|---|
| Revenue | $10,990 | $10,163 |
All figures are in millions of dollars,
except EPS, and were translated using
the Federal Reserve Board’s average rate
of exchange in 2018: EUR 1.1817 = $1.00
Outcomes Creativity Index Score: 96
Manny Awards – 14
Cannes Lions – 30
LIA: Health & Wellness – 8
Clio Health – 9
One Show: HW&P – 3
MM&M Awards – 11
Global Awards – 13
Creative Floor Awards – 8
For Boehringer Ingelheim, 2018 was a successful financial year. Family-owned since 1885, the company concentrates on human pharmaceuticals and animal health.
According to its leaders, at almost €1 billion – 9 percent more than in 2017 – 2018 investments in tangible assets were higher than ever before. “In 2018, the return on net sales increased from 19.3 to 19.8 percent, while our equity ratio rose from nearly 38 percent to 40 percent. Thus we are also a very healthy company from a financial point of view,” says Michael Schmelmer, member of the Board of Managing Directors responsible for Finance.

“We want to make a significant contribution towards a superior treatment of cancer. In addition, we are conducting research, among others, into fibrotic, metabolic, and immunological diseases. Our research pipeline is well-filled,” says Hubertus von Baumbach, Chairman of the Board of Managing Directors, Boehringer Ingelheim GmbH.
The average number of employees in all regions increased about 2 percent to a total of 50,370.
Looking to the future, in August 2019 Boehringer Ingelheim announced changes to its Board of Managing Directors.
Allan Hillgrove, member of the Board of Managing Directors responsible for the Human Pharma and the Biopharma business, will step down from the board and retire at the end of 2019 after 37 years. Hillgrove joined Boehringer Ingelheim in 1982 in Australia as a sales rep in Animal Health. Throughout his career, he held numerous Human Pharma management positions in Australia and corporate, including head of Corporate Department Respiratory Marketing. He was appointed to the Board of Managing Directors in 2013, and took the added responsibility for the Biopharma Business in 2018.
The shareholders have appointed Dr Carine Brouillon to the Board of Managing Directors, effective Jan. 1, 2020, as Hillgrove’s successor. Since she joined Boehringer Ingelheim in 2018, Dr. Brouillon has been leading the Therapeutic Areas for prescription medicines. She has an academic background in natural science and economics as well as broad international expertise in product, marketing, and commercial strategies. Executives say Dr. Brouillon will continue to align the human pharmaceutical business to future needs of patients.
Also at the end of 2019, Dr Joachim Hasenmaier will retire and step down as member of the Board of Managing Directors and from his responsibility for the Animal Health business. Hasenmaier joined Boehringer Ingelheim in 2001 as Head of the Animal Health Division. Executives say he led the business into a period of successful growth, which included acquisitions such as parts of Fort Dodge, an animal health company, in 2009. In 2012, he was appointed as member of the Board of Managing Directors with responsibility for the two businesses, Animal Health as well as Consumer Health Care.
Jean Scheftsik de Szolnok is member of the Board of Managing Directors and new head of the Animal Health Business Unit, effective Jan. 1, 2020.
De Szolnok joined the corporation in 1987. Since September 2017, he has been the country managing director of France. In this role, he was instrumental in managing the Merial integration and further development of the Lyon site as a research, development, and production site for the animal health business. Before that, he held various leadership positions in Germany as well as in Canada, Belgium, and France.
To ensure continuous success in all aspects of organizational and employee development, as well as its attractiveness as an employer, Boehringer Ingelheim is moving the responsibility for the overall people strategy under the leadership of Hubertus von Baumbach, the chairman of the Board of Managing Directors, as of Oct. 1, 2019.
Because new technologies and digital transformation have moved many tasks of enabling corporate functions closer together, some human resources activities will become part of the responsibility of the board’s Division Finance. As a result, Dr Andreas Neumann decided to step down from the Board of Managing Directors at the end of September. He held the position of general counsel from 2011 until 2015. During this time, the global compliance concept and a corresponding organization was established. Neumann then was appointed to the Board of Managing Directors with responsibility for human resources.
“During the tenure of Andreas Neumann, the global framework of OUR FOCUS was created, a central building block for the development and transformation of our organization,” says Christian Boehringer, chairman of the Shareholders‘ Committee. “At the same time, Diversity & Inclusion as well as people development have been important topics of his leadership. The shareholders would like to express their highest appreciation for his many years of dedication to Boehringer Ingelheim, and wish him and his family all the best for the years to come.”
Financial performance
Boehringer Ingelheim recorded net sales of €17.49 billion ($20.68 billion) in the 2018 financial year, which corresponds to a decrease of 3.1 percent as compared with the previous year. Executives say the exchange rate developments on the foreign exchange markets and the associated exchange rate effects had a negative impact on sales growth in the biggest markets.
In the 2018 financial year, Boehringer Ingelheim achieved the majority of its sales in the Americas (46 percent) and Europe (30 percent). Executives say the region of Asia/Australia/Africa (AAA) is of strategic significance for the group’s future growth, making up 24 percent of company sales. The three biggest market – the United States, Japan and Germany – accounted for 52 percent of sales in 2018.
At €12.6 billion ($14.89 billion), human pharmaceuticals contributed 72 percent of total net sales in 2018, management says, adding that foreseeable declines in net sales – due to expiring patents for innovative medicines – were more than compensated for. Adjusting for currency, the sector grew 5.1 percent. Revenue from the global licensing business was lower than in the previous year and have reduced the overall rate of growth in this business area to 3.3 percent.
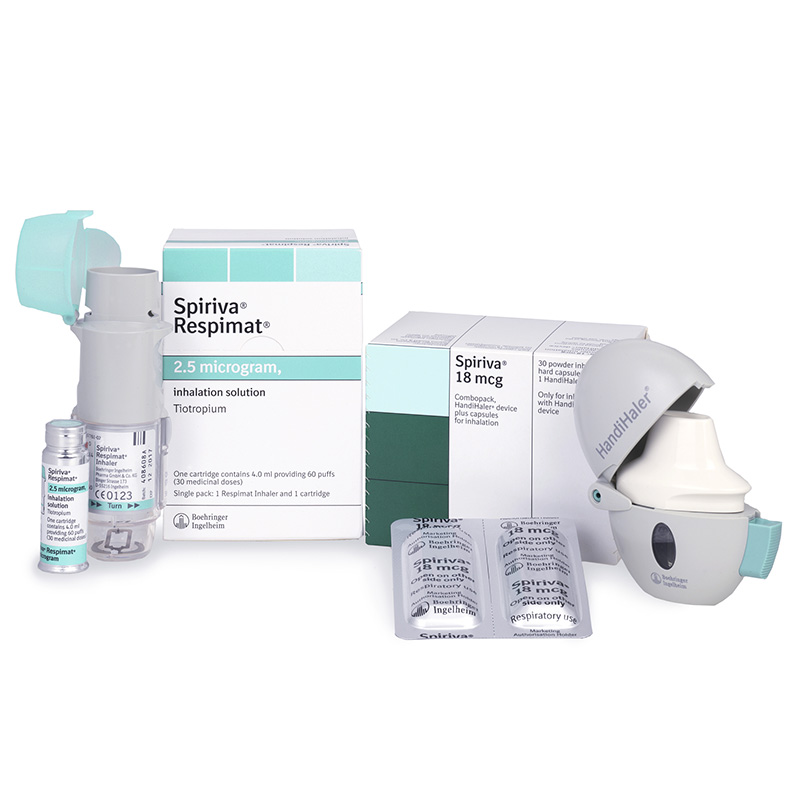
The company’s top four products in sales during 2018 were Spiriva, Pradaxa, Jardiance, and Trajenta. As in previous years, the respiratory medicine Spiriva achieved the highest net sales contributions with €2.4 billion ($2.85 billion), down 14.3 percent, followed by the anticoagulant Pradaxa with €1.49 billion ($1.76 billion), up 3.3 percent; the type 2 diabetes medicine Jardiance, with €1.46 billion ($1.73 billion), up 44.9 percent; and the type 2 diabetes medicine Trajenta with €1.4 billion ($1.65 billion), up 4.8 percent. The idiopathic pulmonary fibrosis drug Ofev generated 2018 sales of €1.1 billion ($1.3 billion), compared to €920 million ($1.09 billion) during 2017.
The company spent €3.16 billion ($3.74 billion) on R&D in 2018, 2.8 percent more than in 2017. R&D for human pharmaceuticals, at €2.78 billion ($3.29 billion), corresponded to a 22.1 percent share of human pharmaceuticals’ net sales.

Spiriva remains Boehringer Ingelheim’s leading drug, though 2018 sales declined 14.3 percent to €2.41 billion ($2.85 billion).
For the first six months of 2019, management reported that the company generated total net sales of around €9.3 billion ($10.99 billion) compared with €8.6 billion ($10.16 billion) in the same period last year. According to executives, adjusted for currency effects, this gives year-on-year growth of 4.6 percent. Human pharmaceuticals contributed to robust organic growth, but net sales in the animal health business area were down slightly due to the outbreak of African swine fever in Asia. The results in biopharmaceutical contract manufacturing were adversely impacted by unfavorable phase effects.
“We are pleased with our half-year result. Boehringer Ingelheim is growing organically in a sustainable and stable manner,” von Baumbach says.
With human pharmaceuticals, Boehringer Ingelheim’s largest business, the company achieved first-half 2019 net sales of €6.8 billion ($8.04 billion) compared with €6.1 billion ($7.21 billion) in first-half 2018. According to executives, the main growth driver is the diabetes portfolio with Jardiance, sales of which increased by a currency-adjusted 44.8 percent to €1 billion ($1.18 billion).
In animal health, Boehringer Ingelheim’s focus is on innovative vaccines, antiparasitic medicines, and other therapy solutions for livestock and pets. In 2018, the company’s four best-selling animal-health products were the antiparasitic medicines Nexgard, Frontline and Heartgard plus the vaccine Ingelvac Circoflex. Net sales of €4 billion ($4.73 billion) represented 23 percent of total net sales.

The type 2 diabetes medicine Jardiance generated 2018 sales of €1.46 billion ($1.73 billion), up 44.9 percent.
Two years after acquiring the Merial animal health business from Sanofi (see profile on page 68), the animal health unit has achieved significant net sales growth, with a currency-adjusted rate of 5.6 percent, while simultaneously undertaking integration efforts.
“We have focused on providing our customers with continuous supply from day one. As a result, we have achieved good growth and the technical integration is successfully completed,” von Baumbach says. Executives say the innovation potential in animal health, where it interrelates with human pharmaceutical research, deserves particular attention.
In first-half 2019, the animal health business generated net sales of around €2.1 billion ($2.48 billion), about the same as in same-period 2018. Adjusted for currency effects, this results in a decrease of 1.7 percent.
Nexgard sales rose by 13.8 percent to €395 million ($467 million) on a currency-adjusted basis and was the best-selling animal-health product for Boehringer Ingelheim. Vaccines for livestock were strongly influenced by the African swine fever outbreak in China and Southeast Asia, where millions of pigs are being culled to halt the disease. Accordingly, net sales of the swine vaccine Ingelvac Circoflex declined by 23.3 percent to €117 million ($132 millio8) on a currency-adjusted basis. Management says despite these challenges, the region remains an important growth market in animal health for Boehringer Ingelheim.

Sales of Trajenta/Jentadueto for type 2 diabetes went up 4.8 percent in 2018 to €1.4 billion ($1.65 billion).
According to company executives, Boehringer Ingelheim’s biopharmaceuticals business in 2018 maintained its lead position in the contract manufacturing segment, providing 4 percent of overall net sales. “The order situation has continued to develop positively and provided for a high level of capacity utilization in biopharmaceutical production,” management says.
Net sales in biopharmaceutical contract manufacturing were below the previous year’s level, resulting in net sales of €273 million ($323 million) in the first half of 2019 (2018: €298 million/$352 million). The decrease is mainly attributable to unfavorable phase effects. The focus is on expanding production capacities in Europe, the United States and Asia, because the significance of biopharmaceutical medicines continues to grow worldwide.
In 2019, executives say Boehringer Ingelheim is expecting slight growth in net sales and further intensive investment activities on a comparable basis.
“In Europe alone we are planning investments of more than €3 billion ($3.55 billion) within the next five years,” von Baumbach says. “The key precondition for this are competitive overall conditions at our European sites.”
The company has been investing in strategic acquisitions. In July, Boehringer Ingelheim announced that the company had acquired all shares of AMAL Therapeutics SA, a private Swiss biotechnology company focused on cancer immunotherapy and advancing first-in-class therapeutic cancer vaccines derived from its technology platform KISIMA. AMAL’s lead vaccine ATP128 is currently developed for stage IV colorectal cancer and was slated to begin first-in-human trials later on in the month. Boehringer Ingelheim plans to develop new therapies by combining assets from its cancer immunology portfolio with AMAL’s proprietary KISIMA immunization platform.
“Acquiring AMAL is part of Boehringer Ingelheim’s long-term strategy to enhance our existing position as an innovator of novel cancer therapies, including immuno-oncology treatments, which leverage cutting-edge scientific discoveries and their applications,” says Michel Pairet, member of Boehringer Ingelheim’s Board of Managing Directors with responsibility for the company’s Innovation Unit. “We want to pioneer new paradigms of biology-based care for cancer patients, and the technologies and expertise developed at AMAL are critical to our efforts.”
The total transaction could amount up to €325 million ($384 million), and comprises an upfront payment as well as contingent clinical, development and regulatory milestones plus up to €100 million ($118 million) if certain commercial milestones are hit.
According to executives, the AMAL acquisition, along with the 2018 acquisition of Vira Therapeutics and in-license of OSE Immunotherapeutics’ SIRP-alpha targeting antibody, significantly strengthens Boehringer Ingelheim’s strategic focus on immune cell-directed therapies. “By combining its world-class in-house research and development with that of highly innovative biotechnology companies, Boehringer Ingelheim is developing innovative immuno-oncology therapies and accelerating the delivery of the next-generation of cancer treatments,” executives say.
In March, Boehringer Ingelheim announced that the company had acquired ICD Therapeutics. The acquisition includes rights to ICD’s innovative MacroDel biologics-delivery platform. Boehringer Ingelheim will employ this platform for the development of novel therapeutics in collaboration with nanoPET Pharma GmbH, a former shareholder of ICD Therapeutics.
“Boehringer Ingelheim’s collaboration with nanoPET Pharma has the potential to eliminate the hurdle that many cancer biologics face: getting access to targets inside tumor cells,” says Norbert Kraut, Ph.D., global head of Cancer Research, Boehringer Ingelheim. “We will use ICD’s MacroDel technology to develop first-in-class potential drug candidates for intracellular targets across a variety of tumor types, for the benefit of patients who so far have no or only inadequate treatment options.”
R&D and collaborations
For the total of 90 projects in all phases of the research process, executives say the goal is for 75 percent of them to be either the first molecules in their active ingredient class or the first in a new therapeutic area. In 2019, that was true of 67 percent of the company’s current R&D projects.
In oncology, the focal points are cancers of the lung, stomach and intestine, while in fibrotic diseases the focus is on systemic scleroderma with interstitial lung disease. In metabolic diseases, non-alcoholic steatohepatitis is the main focus of research.
In immunology, research is giving attention to chronic inflammatory diseases of the skin and intestine. Other projects address diseases of the central nervous system, such as Alzheimer’s and schizophrenia, obesity and retinopathy.
“We want to make a significant contribution towards a superior treatment of cancer,” von Baumbach says. “In addition, we are conducting research, among others, into fibrotic, metabolic, and immunological diseases. Our research pipeline is well-filled.”
Boehringer Ingelheim also actively seeks research collaborations, with “more than 150 cooperations at every level of the research value chain,” executives say. Additionally, the company has set – and reached – a target that a third of the product pipeline should be based on in-licensed mechanism or molecules.
In July, Boehringer Ingelheim and Yuhan Corp. announced a collaboration and license agreement for the development of a first-in-class dual agonist for the treatment of NASH and related liver diseases that combines GLP-1 and FGF21 activity in one molecule. The collaboration brings together Yuhan’s expertise in FGF21 biology, obesity, and NASH with Boehringer Ingelheim’s pharmaceutical expertise and commitment to bringing innovative medicines to patients with cardiometabolic diseases.
Preclinical evidence suggests high efficacy, when combining the gut-derived hormone GLP-1 with FGF21. The dual agonist (GLP1R/FGF21R agonist) is expected to reduce liver cell injury and hepatic inflammation by resolution of steatohepatitis as well as having direct antifibrotic effects and complements Boehringer Ingelheim’s R&D portfolio in NASH adding another potential first-in-class opportunity.
“We are excited about this new opportunity which adds to our longstanding and successful partnership with Yuhan Corporation,” Pairet says. “This partnership brings us closer to next generation treatments for patients with NASH.”
In September, Boehringer Ingelheim and Lupin Ltd. announced a licensing, development and commercialization agreement for Lupin’s MEK inhibitor compound LNP3794 as a potential targeted therapy for patients with difficult-to-treat cancers. The partnership aims to develop LNP3794 in combination with one of Boehringer Ingelheim’s innovative KRAS inhibitors for patients with gastrointestinal and lung cancers harboring a broad range of oncogenic KRAS mutations.
“The licensing of Lupin’s novel MEK inhibitor enables us to pair with our innovative KRAS inhibitors to develop new combination treatment concepts providing more effective and durable responses for patients with cancers driven by activated KRAS who currently have limited treatment options available,” says Norbert Kraut, Ph.D., head of Global Cancer Research at Boehringer Ingelheim. “We believe this collaboration will significantly strengthen our KRAS program. We have developed comprehensive approaches to successfully tackle the oncogenic KRAS–RAF–MEK–ERK pathway from the ground up and this partnership is another key building block in our long-term strategy to bring novel treatments to patients in our quest to defeat intractable cancer types.”
Also in September, Boehringer Ingelhein and Inflammasome Therapeutics Inc. announced a joint development and license agreement to develop up to three therapies for patients with retinal diseases. By combining Inflammasome’s unique intravitreal (IVT) drug-delivery technologies with Boehringer Ingelheim’s compounds from its retinal disease pipeline portfolio, Boehringer Ingelheim aims to develop novel therapies for retinal diseases.
The collaboration between Boehringer Ingelheim and Inflammasome aims to address the challenge posed by retinal diseases by using Inflammasome’s technology to deliver therapeutics in a biodegradable gel formulation into the eye. “Boehringer Ingelheim is looking forward to developing Inflammasome’s novel technology for the delivery of our first-in- class retinal disease compounds working jointly with Inflammasome’s highly experienced scientific team,” says Clive R. Wood, PhD, senior corporate VP, Discovery Research at Boehringer Ingelheim. “This will enable us to develop a broad range of novel therapy options for the many patients with retinal diseases waiting urgently for better and new therapy options.”
Inflammasome is entitled to receive up to $160 million in up-front, R&D support and milestone gated development payments as well as tiered royalties based on future commercial sales of developed products and other milestones due on commercialization.
In July, Boehringer Ingelheim and Bridge Biotherapeutics Inc. announced that they are entering into a new collaboration and license agreement with the goal of developing Bridge Biotherapeutics’ autotaxin inhibitor BBT-877 for patients with fibrosing interstitial lung diseases, including idiopathic pulmonary fibrosis. BBT-877 is in Phase I clinical studies and is anticipated to enter Phase II testing within the next 12 months.
Both companies will initially focus on developing the compound for the treatment of IPF, an area of high unmet medical need and one of the key focus areas of Boehringer Ingelheim. Boehringer Ingelheim has developed Ofev, an antifibrotic drug shown to slow disease progression by reducing lung function decline and currently approved for the treatment of idiopathic pulmonary fibrosis in more than 70 countries around the world including the United States, the EU and Japan.
Idiopathic pulmonary fibrosis is a rare, debilitating and fatal lung disease affecting about 3 million people worldwide. IPF causes progressive scarring of the lungs, resulting in continual and irreversible deterioration in lung function and breathing difficulties.
BBT-877 inhibits autotaxin, an enzyme mediating a key pro-fibrotic event in multiple cell types. It has shown a promising safety and efficacy profile in pre-clinical models for fibrosing interstitial lung diseases and potential for combination with the current standard of care.
“We look forward to working with the team at Bridge Biotherapeutics to develop a new treatment option for patients with IPF,” Pairet says. “This new collaboration complements our growing pipeline in fibrosing interstitial lung diseases and is a sign of our determination to bring the next generation of treatment options to these patients.”
In August, Boehringer Ingelheim and The University of Texas MD Anderson Cancer Center announced a new multi-year partnership to conduct collaborative research to rapidly advance therapies for various types of cancers, including gastrointestinal and lung cancers. The establishment of a joint Virtual Research and Development Center will enable effective data sharing and analysis between the organizations.
According to BI executives, the partnership is built on a flexible framework, allowing for projects to enter at different stages (research, development and/or clinical stage) over several years. The partnership combines the unique patient-driven drug-development capabilities of MD Anderson’s Therapeutics Discovery division with the innovative pipeline of novel medicines from Boehringer Ingelheim.
The Virtual Research and Development Center focuses on the development of potential new treatments including KRAS inhibition concepts, as mutations in the KRAS gene are common in cancers, specifically in certain types of lung and gastrointestinal cancers; and a TRAILR2 agonistic antibody, with the potential to selectively induce cancer cell death (apoptosis).
“We could not have chosen a better partner with all its research, translational and clinical expertise in lung and gastrointestinal cancers. Together, we hope to transform the treatment landscape for these diseases by tackling their root causes and drivers, that have so far remained elusive, exploring new and smart ways of killing cancer cells,“ says Dr. Victoria Zazulina, corporate VP and Global Head of Oncology, Medicine, at Boehringer Ingelheim. “Our innovative oncology pipeline coupled with strong partnerships like this will contribute to unravelling the complexities of these diseases and bringing innovative solutions to people with various types of cancers.”
In recent pipeline progress, the company gained approval from FDA in September for Ofev as the first medicine to slow the rate of decline in pulmonary function in patients with systemic sclerosis associated interstitial lung disease (SSc-ILD). Initially approved and launched in the United States during October 2014 for the treatment of patients living with IPF, Ofev has been shown to slow progression of the lung disease by reducing the annual rate of decline in lung function, as measured by forced vital capacity (FVC).
The September 2019 approval was based on results of SENSCIS, a Phase III double-blind randomized, placebo-controlled trial, that involved 576 patients from 194 trial sites across 32 countries. The primary endpoint was the annual rate of decline in FVC in patients with SSc-ILD. Results shows that Ofev slowed the loss of pulmonary function by 44 percent (41 mL/year) in patients with SSc-ILD relative to placebo, as measured by FVC over 52 weeks.
“This is the first FDA-approved therapy to slow the rate of decline in pulmonary function for systemic sclerosis associated interstitial lung disease and provides hope to patients and their loved ones facing this devastating disease,” says Thomas Seck, M.D., senior VP, Medicine & Regulatory Affairs, Boehringer Ingelheim Pharmaceuticals Inc. “This approval is supported by positive evidence from the Phase III study that showed Ofev significantly slowed the progression of lung function decline in this patient population and exemplifies Boehringer Ingelheim’s dedication to the rare disease community.”
In June, Boehringer Ingelheim and Eli Lilly and Co. announced that FDA has granted Fast Track designation to empagliflozin for the reduction of the risk of cardiovascular death and hospitalization for heart failure in people with chronic heart failure. Empagliflozin is marketed as Jardiance.
This designation is for the EMPEROR program, which consists of the EMPEROR-Reduced and EMPEROR-Preserved studies. These studies will evaluate the effect of empagliflozin on cardiovascular death and hospitalization for heart failure in adults with chronic heart failure with reduced or preserved ejection fraction.
“Heart failure is a leading cause of hospitalization, with more than 1 million admissions annually just in the US and Europe. Yet, there are limited treatment options for people living with this debilitating condition,” says Waheed Jamal, MD, corporate VP and head of CardioMetabolic Medicine, Boehringer Ingelheim. “The FDA Fast Track designation for empagliflozin is an important step forward in addressing this unmet need in the U.S., and we look forward to working closely with the FDA as we explore the potential for empagliflozin to improve outcomes for adults with chronic heart failure.”
In May, Boehringer Ingelheim and Gubra, a privately owned biotech company based in Denmark, announced a second collaboration and license agreement for the development of novel poly-agonist peptides to treat obesity. The collaboration brings together Gubra’s expertise in the design, synthesis, pharmaceutical characterization, and in vivo testing of therapeutic peptides with Boehringer Ingelheim’s R&D expertise.
This second joint research and development program between Boehringer Ingelheim and Gubra builds on a successful ongoing collaboration in the obesity field, which was launched in 2017, and which has already achieved important milestones. It contributes to the growing Boehringer Ingelheim research and development stage portfolio in the obesity field.
“We look forward to expanding our productive collaboration with the Gubra team,” Wood says. “I anticipate that these programs will help us bring much needed new treatment options for obesity patients.”