The U.S. Food and Drug Administration on Oct. 12 allowed British American Tobacco Plc to market the company’s Vuse Solo e-cigarettes and tobacco-flavored pods, the first-ever vapor product to get clearance from the health regulator.
More than 55 million people worldwide are living with dementia, a neurological disorder that robs them of their memory and costs the world $1.3 trillion a year, the World Health Organization (WHO) said on Sept. 2.
Biotech company Psycheceutical Inc. of Ft. Lauderdale, Fla., was issued two patents by the United States Patent and Trademark Office for what it calls a “precision duplicity” approach to delivering psychedelic drugs to patients.
Philip Morris continues to expand the tobacco giant’s ‘Beyond Nicotine’ strategy by acquiring Michigan-based OtiTopic, a company developing inhalable acetylsalicylic acid (ASA) treatment for acute myocardial infarction.
Light-to-moderate alcohol consumption is linked to a reduced risk of heart attack, stroke and death among those with heart disease, according to a study published in the journal BMC Medicine on July 26.
Avenue Therapeutics Inc. said on June 14 the U.S. Food and Drug Administration had once again declined to approve the company’s non-opioid painkiller tramadol, sending the drugmaker’s shares lower in trading.
Several biopharmaceutical companies submitted New Drug Applications (NDAs) to the U.S. Food and Drug Administration in recent weeks, covering treatment indications ranging from an opioid overdose to bipolar disorder and rare disease.
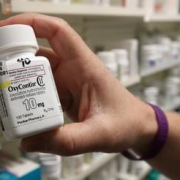
OxyContin maker Purdue Pharma pleads guilty to criminal charges
Addictions, Bankruptcy, Business, Criminal Charges, District Judges, Doctors, Former Blockbusters, Investigations, Kickbacks, New Jersey, OxyContin, Painkillers, Plea Deals, Purdue Pharma, Therapeutics, U.S. Justice Department, U.S. Opioid CrisisPurdue Pharma LP pleaded guilty to criminal charges over the handling of the company’s addictive prescription painkiller OxyContin, capping a deal with federal prosecutors to resolve an investigation into the drugmaker’s role in the U.S. opioid crisis.
Walmart Inc. filed a lawsuit against the federal government, seeking clarity on the roles and legal responsibilities of pharmacists and pharmacies in filling opioid prescriptions.
O.C Suboxone understands the severity of opioid use disorder and is working to raise awareness on healthier alternatives to treat chronic pain.