AbbVie bolstered the company’s neuropsychiatric and neurodegenerative portfolio with the acquisition of Belgium-based Syndesi Therapeutics in a deal valued at up to $1 billion.
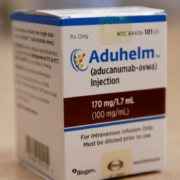
Lilly and Roche expressed their criticism of the draft guidance from the U.S. Centers for Medicare & Medicaid Services (CMS) for Biogen’s Alzheimer’s drug Aduhelm (aducanumab).
What’s ahead in Biden’s year 2?
ACA, Alzheimer’s disease, Approvals, Centers for Medicare and Medicaid Services (CMS), Coalition for Healthcare Communication (CHC), Congress, Coronavirus Disease (COVID-19) Pandemic, Department of Health and Human Services (HHS), FDA, FDA Commissioner, February 2022, Issue Archives, Joe Biden, Jon Bigelow, PDUFA, TherapeuticsEach new president enters the White House with big dreams and unique challenges. For President Joe Biden, 2021 was dominated by the evolving COVID-19 pandemic, an historically difficult transition of power, and a focus on packing an ambitious combination of economic relief, infrastructure investment, and social spending initiatives into a handful of multi-trillion dollar omnibus bills to push through a tightly-divided Congress.
Shares of Cassava Sciences were up more than 5% in premarket trading on February 11 after the U.S. Food and Drug Administration denied a Citizen Petition filed during 2021 on behalf of short-selling clients who sought to suspend the company’s Alzheimer’s clinical trials.
Alzheon posted positive results from the company’s Phase II biomarker trial on ALZ-801 (valiltramiprosate) in patients diagnosed with neurodegenerative disorders such as Alzheimer’s disease.
Scientists believe that Alzheimer’s disease is a potential cause of not just beta-amyloid accumulation, but also immune system dysfunction.
On the strength of data from Alzamend Neuro’s Phase I study of its lead compound AL001 being developed for the treatment of Alzheimer’s disease, the company is looking to commence a Phase II multiple ascending dose study for that indication during the second quarter of 2022.
While the Centers for Medicare & Medicaid Services undergoes the comment period on its national coverage decision for Biogen’s controversial Alzheimer’s drug Aduhelm (aducanumab), Biogen and its partner company Eisai released additional details about the Phase IV post-marketing study of the drug.
South San Francisco-based Cortexyme Inc. received a letter from the U.S. Food and Drug Administration on January 25 placing a full clinical hold on the Investigational New Drug application for the company’s lead clinical asset atuzaginstat, which is in development for the treatment of Alzheimer’s disease.
The U.S. government’s Medicare program on January 11 said it plans to cover Alzheimer’s treatments including Biogen Inc.’s Aduhelm, with some conditions.

 © AbbVie Inc. All rights reserved.
© AbbVie Inc. All rights reserved.