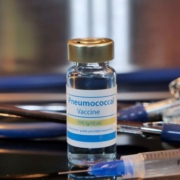
Global biopharmaceutical firm Merck announced that the company’s Phase III trial for a potential treatment for pneumococcal disease in infants succeeded in meeting key safety, tolerability, and immunogenicity endpoints in the first 30 days.
Shares of Aprea Therapeutics plunged after the Boston-based company announced a late-stage cancer combination treatment failed to meet the primary endpoint of complete remission rate.
The U.S. Food and Drug Administration approved MorphoSys and Incyte’s Monjuvi (tafasitamab-cxix) in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant.
2016 Annual Report: Top 10 Pipelines
"Bad" LDL Cholesterol, Alzheimer's Diseases, Approvals, Big Pharma, Biologics License Application (BLA), Biosimilars, Biotech, Biotech/Biopharma, Biotechnology, Bispecific CD19-directed CD3 T cell engager (BiTE) antibodies, Blockbusters, Blood Cancers, Breakthrough Designation, Breakthrough Therapy, Breakthrough Therapy Designation, Breakthrough Therapy Designation, Cells, Chimeric Antigen Receptor T Cell Therapy (CART), Cholesterol, Chronic Lymphocytic Leukemia, Clinical Trials, Collaboration, Collaborations, Collaborations, Cystic Fibrosis, Deals, Diabetes, DNA, EGFR Inhibitors, Europe, European Commission, FDA, FDA/Regulatory, February 2016, Glucagon-Like Peptide-1 (GLP-1) Analogs, Immune Response, Immune System, Immune Systems, Immunotherapies, Influenza, Inhalers, Issue Archives, Mantle Cell Lymphoma (MCL), Monoclonal Antibodies, Multiple Myeloma, NDA, Neuroscience, New Drug Application (NDA), New Molecular Entities, Non-Small Cell Lung Cancer, Oncology, Pain, Parkinson's, Parkinson's Disease, Plaque Psoriasis, Potential Blockbusters, Psoriasis, R&D, Sales, SGLT2 Inhibitors, Special Reports, Specialty Therapy, Technology, TGF-Beta Inhibitors, The New England Journal of Medicine (NEJM), Therapeutics, Top 10 Pipelines, Transforming Growth Factor-beta (TGFb), Type 1 Diabetes, Type 2 Diabetes, Vaccines, Waldenstrom’s macroglobulinemiaThe pharma industry’s R&D concentration has been shifting towards specialty therapy areas as research and development returns decline for some leaders.