The U.S. FDA rejected Cambridge, Massachusetts-based Akebia Therapeutics’ New Drug Application (NDA) for vadadustat, a therapeutic for anemia due to chronic kidney disease (CKD).
A trial assessing an experimental AstraZeneca cancer drug was placed on clinical hold due to safety concerns. The trial pause comes two years after Amgen was also forced to pause a study of a drug within the same class.
A safety concern forced Takeda Pharmaceutical to suspend the dosing of patients in two Phase II studies of an experimental treatment for narcolepsy. The clinical trial halt came one day after the company forged a gene therapy agreement with Selecta Biosciences valued at $1.1 billion.
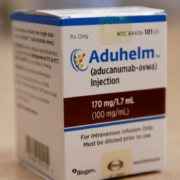
The U.S. Veterans Health Administration will not include Aduhelm, the $56,000-a-year Alzheimer’s drug made by Biogen Inc. and Eisai Co. Ltd., on the VHA’s list of approved products due to a lack of evidence that the medicine is effective as well as safety concerns, the agency said on Aug. 11.
Novartis cut three Phase III trials of the eye therapy Beovu (brolucizumab) after the studies showed higher rates of intraocular inflammation with frequent dosing intervals of the therapy.
U.S. drugmaker Eli Lilly and Co. said the government-sponsored clinical trial of the company’s Covid-19 antibody treatment similar to one taken by U.S. President Donald Trump was paused because of a safety concern.
The adverse events that led to a pause in trials evaluating AstraZeneca Plc’s Covid-19 vaccine candidate may not have been associated with the vaccine itself, according to a document outlining participant information that was posted online by the Oxford University.
The National Institutes of Health (NIH) raised concern about the safety of AstraZeneca’s coronavirus vaccine as the U.S. Food and Drug Administration is weighing whether or not to resume testing in the United States following a global pause in the Phase III trial for safety reasons.
Although it’s increasingly likely that one of the Covid-19 vaccines being tested will be approved or receive Emergency Use Authorization (EUA) before the end of 2020, experts are increasingly warning that widespread distribution may take a much longer time.
AstraZeneca Plc paused global trials, including large late-stage studies, of the company’s experimental coronavirus vaccine because of an unexplained illness in a study participant.