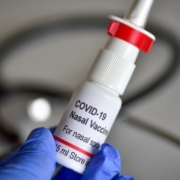
Meissa Vaccines’ intranasal live attenuated chimeric virus-based vaccine for SARS-CoV-2 (and its variants), which was cleared for Phase I trials, employs codon deoptimization to evoke a stronger-than-usual immune response.
BioSpace Global Roundup: Ipsen Wins Fast Track Designation for Cancer Drug and More
3D, Antibody-Drug Conjugates (ADCs), Business, Clinical Trials, Collaborations, Coronavirus Disease 2019 (COVID-19), Data, E. Coli, Fast Track Designation, FDA, Gene Editing, Joint Ventures, Licensing, Metastatic pancreatic cancer, Neurological Disorders, R&D, Series A, Small cell lung cancer, Staphylococcus aureus infections, Therapeutics, Ulcerative Colitis, VaccinesParis-based Ipsen secured Fast Track designation from the U.S. Food and Drug Administration for Onivyde (irinotecan liposome injection) for study patients with small cell lung cancer (SCLC) who progressed following a first-line platinum-based regimen.
FDA Awards Fast Track Designation to Multiple Non-Covid-19 Candidates
Acid Disorders, Acute repetitive seizures, Coronavirus Disease (COVID-19) Pandemic, Fast Track Designation, FDA, Gene Therapy, Hypogammaglobulinemia, Imaging Agents, Infections and Myelokathexis) syndrome, Methylmalonic Acidemia (MMA), MPS Type VI, Non-Small Cell Lung Cancer (NSCLC), Phenylketonuria (PKU), Primary Mitochondrial Myopathy (PMM), Solid Tumors, WHIM (WartsWhile the world has largely been focused on the development of vaccines and therapeutics for Covid-19, the U.S. Food and Drug Administration has remained busy lining up potential approvals of medications for other diseases and illnesses.
Aptinyx Jumps Following Positive Phase II PTSD Study Results Published: Oct. 20, 2020 By Alex Keown BioSpace Shares of Aptinyx skyrocketed more than 75% after the company reported its […]
Galapagos and Servier reported that their ROCELLA Phase II clinical trial of GLPG1972/S201086 failed to meet the study’s primary endpoint in osteoarthritis knee repair.
Pfizer snagged Fast Track designation from the U.S. Food and Drug Administration for the company’s Duchenne muscular dystrophy (DMD) gene therapy treatment, PF-06939926.
ALX Oncology is partnering with pharma giant Merck to evaluate a combination of the CD47 inhibitor ALX148 with the blockbuster Keytruda as a potential treatment for patients with head and neck squamous cell carcinoma (HNSCC).
Orphazyme’s Rare Lipid Disorder Drug Gets FDA Priority Review
Accepted NDA, Clinical Data, Clinical Trials, Fast Track Designation, FDA, FDA/Regulatory, Heat-Shock Proteins (HSPs), Lipid disorders, Niemann-Pick C Disease (NPC), Primary Endpoints, Priority Review Status, R&D, Rare Genetic Diseases, Secondary EndpointsThe U.S. Food and Drug Administration accepted Copenhagen, Denmark-based Orphazyme’s New Drug Application (NDA) for arimoclomol for the treatment of Niemann-Pick disease Type C.
August is a busy month on the U.S. Food and Drug Administration)’s calendar, including a target action date for Bristol Myers Squibb’s supplemental Biologics License Application for Opdivo (nivolumab) plus Yervoy (ipilimumab), dosed concomitantly with a limited course of chemotherapy, for the first-line treatment of metastatic or recurrent non-small cell lung cancer with no EGFR or ALK genomic tumor aberrations.
The U.S. Food and Drug Administration approved MorphoSys and Incyte’s Monjuvi (tafasitamab-cxix) in combination with lenalidomide for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma not otherwise specified, including DLBCL arising from low grade lymphoma, and who are not eligible for autologous stem cell transplant.