The Chinese government halted the recommendation of an herbal supplement used as a traditional Chinese treatment for mild cases of COVID-19.
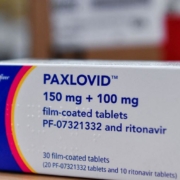
China’s medical products regulator said on February 12 it has given conditional approval for Pfizer’s COVID-19 drug Paxlovid, the first oral pill specifically developed to treat the disease to be cleared in the country.
China welcomed the first CAR T-cell therapy in the country following approval from the National Medical Products Administration.
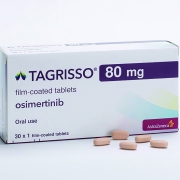
AstraZeneca Plc said on April 14 that China’s health regulator expanded the use of Tagrisso, the British drugmaker’s lung cancer treatment, in patients with a type of lung cancer when diagnosed at an early stage.
China approved a Covid-19 vaccine developed by an affiliate of state-backed pharmaceutical giant Sinopharm on Dec. 31, the country’s first approved shot for general public use as it braces for increased transmission risks over winter.
Junshi’s Checkpoint Inhibitor Ready to Head to Regulators for Throat Cancer
Biologics License Application (BLA), Checkpoint Inhibitors, China, China National Medical Products Administration (NMPA), Clinical Trials, Conditional Approval, FDA/Regulatory, Interim Analysis, Nasopharyngeal Carcinoma (NPC), Progression-Free Survival (PFS), R&D, Throat CancerJunshi Biosciences, based in Shanghai, China, announced that an Independent Monitoring Committee (IDMC) decided the company’s Phase III JUPITER-02 trial had met the pre-specified primary endpoint at the interim analysis.
Sales of Bristol-Myers Squibb Co.’s cancer drug Abraxane were suspended in China based on findings at a third-party manufacturing plant in the United States.