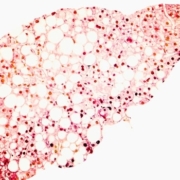
Verantos – a market leader in high-validity real-world evidence generation – announced that along with its research partners, the company accomplished a significant step forward in applying advanced real-world evidence approaches to non-alcoholic steatohepatitis (NASH).
Gaithersburg, Maryland-based Altimmune announced that the U.S. Food and Drug Administration cleared a Phase II clinical trial for the biopharmaceutical company’s investigational obesity-treating drug pemvidutide.
Shares of Madrigal Pharmaceuticals went up in trading after the company announced positive topline data from a Phase III assessment of resmetirom in non-alcoholic fatty liver disease.
Glympse Bio has developed a liquid biopsy with the potential to diagnose and monitor non-alcoholic steatohepatitis (NASH) with significantly higher accuracy and less invasiveness than needle biopsies, according to a late-breaking presentation at the American Association for the Study of Liver Disease (AASLD) annual The Liver Meeting.
Four biotech companies announced Series A rounds: Vita Therapeutics, NuvoAir, Enveda Biosciences, and ImmuneID.
Galectin Therapeutics is one of the only biotech companies with an active Phase IIb/III clinical trial for NASH cirrhosis. Galectin recently launched a site dedicated to its study, NAVIGATE: https://navigatenash.com/. Also, the Journal for ImmunoTherapy of Cancer recently published Phase I clinical research showing belapectin, Galectin Therapeutics’ galectin-3 inhibitor, enhances tumor response in combination with anti-PD-1 therapy. Med Ad News discussed these and other topics with Dr. Pol Boudes, CMO of Galectin.
NGM Biopharmaceuticals’ aldafermin failed to hit the primary endpoint in the company’s Phase IIb ALPINE 2/3 trial for non-alcoholic steatohepatitis (NASH) with stage 2 or 3 liver fibrosis.
France-based Genfit launched NASHnext, a novel, non-invasive diagnostic test for nonalcoholic steatohepatitis (NASH) powered by the company’s proprietary diagnostic technology, NIS4. The test is offered in the United States through Labcorp.
Germany’s Boehringer Ingelheim partnered with Denmark’s Zealand Pharma A/S to study the GLP-1/glucagon dual agonist BI 456906 in two Phase II trials as a potential treatment for adults who are overweight or obese and for adults with non-alcoholic steatohepatitis (NASH).
SubjectWell – the largest risk-free clinical trials marketplace – released statistics on patients’ attitudes toward clinical research, specifically those with liver diseases.