There appears to be a rise in COVID-19 illnesses, driven by Omicron subvariants, which may be better able to evade immunity from vaccines and previous infections and lower public health measures by the public such as masking and social distancing.
Health officials are considering extending the eligibility for a second COVID-19 vaccine booster dose to people under 50 amid a steady rise in cases, with the United States seeing a threefold increase over the past month.
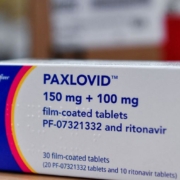
Why Pfizer May Be Holding Paxlovid Close to the Chest
Antivirals, Bloomberg, Clinical Trials, COVID-19 Therapeutics, Drugs for Neglected Diseases Initiative (DNDi), FDA, Hospitalized COVID-19 Patients, Merck, Molnupiravir, Paxlovid, Pfizer, R&D, Symptoms: Coronavirus Disease 2019 (COVID-19), TherapeuticsReportedly, Pfizer is holding the company’s COVID-19 antiviral therapy Paxlovid under tight control. This is a disappointment to numerous investigators who want to test the antiviral combination therapy with other drugs in case the virus develops resistance to the combo.
As U.S. COVID-19 cases rise, so does demand for antivirals
Antivirals, Apple, BNT162b2 (Pfizer and BioNTech), CDC, Connecticut, COVID-19 booster shots, COVID-19 cases, COVID-19 Therapeutics, COVID-19 Vaccines, Delaware, Department of Health and Human Services (HHS), Emergency Use Authorization (EUA), FDA, Health Officials, Hospitalized COVID-19 Patients, Maine, Massachusetts, Merck, Molnupiravir, New York, Northeast, Omicron (B.1.1.529) (South Africa), Omicron BA.2, Paxlovid, Pfizer, Regions, Reuters Tally, Rhode Island, Therapeutics, U.S. government, United States, White HouseRising COVID-19 cases are driving up the use of therapeutics, with Pfizer Inc.’s oral antiviral treatment Paxlovid seeing a 315 percent jump over the past four weeks, U.S. health officials said on May 17.
A chain of events possibly triggered by unrecognized infection with the SARS-CoV-2 coronavirus could be causing the mysterious cases of severe hepatitis reported in hundreds of young children around the world, researchers suggest.
Half of the COVID-19 patients discharged from a Chinese hospital in early 2020 still have at least one symptom two years later, a new study shows. Additionally, new findings suggest patterns of inflammatory proteins in the blood of people with long COVID may someday help guide individualized treatment.
Cancer diagnosis a year before infection not linked to worse outcomes; air travel carries COVID risks
Airline passengers, Airlines, Airports, Cancer, Cancer diagnosis, Coronavirus Infections, COVID-19 Mortality, COVID-19 Studies, COVID-19 transmission, Hospitalized COVID-19 Patients, Journal of Travel Medicine, R&DPatients diagnosed with cancer more than a year before contracting COVID-19 and those not receiving active treatment may be no more vulnerable to worse COVID outcomes than those without cancer, according to a new study. Additionally, researchers warned that passengers are still at risk of coronavirus infection while traveling on airplanes and also in airports.
Obesity may weaken vaccine protection; unvaccinated Omicron patients face risk from variants
AstraZeneca, BNT162b2 (Pfizer and BioNTech), Body Mass Index (BMI), CoronaVac (Sinovac Biotech), COVID-19 immunity, COVID-19 Studies, COVID-19 Vaccinations, COVID-19 Vaccines, Covid-19 Variants, Hospitalized COVID-19 Patients, Immune System, Janssen COVID-19 Vaccine (J&J), Johnson & Johnson, Medical Journals, Messenger RNA (mRNA) Vaccines, mRNA-1273/Moderna COVID-19 Vaccine (Moderna), Nature, Nature Communications, Obesity, Omicron (B.1.1.529) (South Africa), R&D, SARS-CoV-2 virus, Sinovac, South Africa, TurkeySevere obesity may weaken the effectiveness of COVID-19 vaccines in those who have never been infected with the coronavirus, according to a small Turkish study. Additionally, South African researchers have found infection with the Omicron variant of the coronavirus can significantly improve the immune system’s ability to protect against other variants, but only in people who have been vaccinated.
The Omicron variant of the SARS-CoV-2 virus is intrinsically as severe as previous variants, unlike assumptions made in previous studies that it was more transmissible but less severe, a large study in the United States found.
COVID Americas cases up, North American cases up for 5th week – PAHO
Canada, Central America, Coronavirus Cases, Coronavirus Disease (COVID-19) Pandemic, COVID-19 cases, COVID-19 Infections, Hospitalizations, Hospitalized COVID-19 Patients, New Mexico, North America, Pan-American Health Organization (PAHO), Pandemics, Symptomatic COVID-19 Infections, Therapeutics, United StatesCOVID-19 cases in the Americas increased by 12.7 percent last week from the prior week, the Pan American Health Organization (PAHO) said on May 4, as infections continued to rise in Central and North America.