A collaboration between Gilead Sciences and Dragonfly Therapeutics was announced May 2, with an end goal of bringing Dragonfly’s DF7001 natural killer (NK) engager program designed for patients with cancer or inflammatory diseases to fruition.
Regeneron is acquiring Checkmate Pharmaceuticals and entered a clinical trial collaboration with SpringWorks Therapeutics to evaluate REGN5458 in multiple myeloma in combination with nirogacestat.
Opdivo/Yervoy Combo Fails to Meet Endpoints in Head and Neck Cancer
Blockbusters, Bristol Myers Squibb, Checkpoint Inhibitors, Clinical Trial Endpoints, Clinical Trials, Head & Neck Cancer, Head & Neck Squamous Cell Carcinoma (HNSCC), Overall Survival (OS), PD-1/PD-L1 inhibitors, R&D, Secondary EndpointsBristol Myers Squibb shared new details on the global biopharmaceutical firm’s Phase III research on a potential first-line treatment for people who have been diagnosed with recurrent or metastatic squamous cell carcinoma of the head and neck.
Darmstadt, Germany-based Merck KGaA plunked down €188 million (about $226 million) in upfront cash to acquire exclusive global development and commercialization rights to Debiopharm’s oral Inhibitor of Apoptosis Proteins (IAP) antagonist, xevinapant.
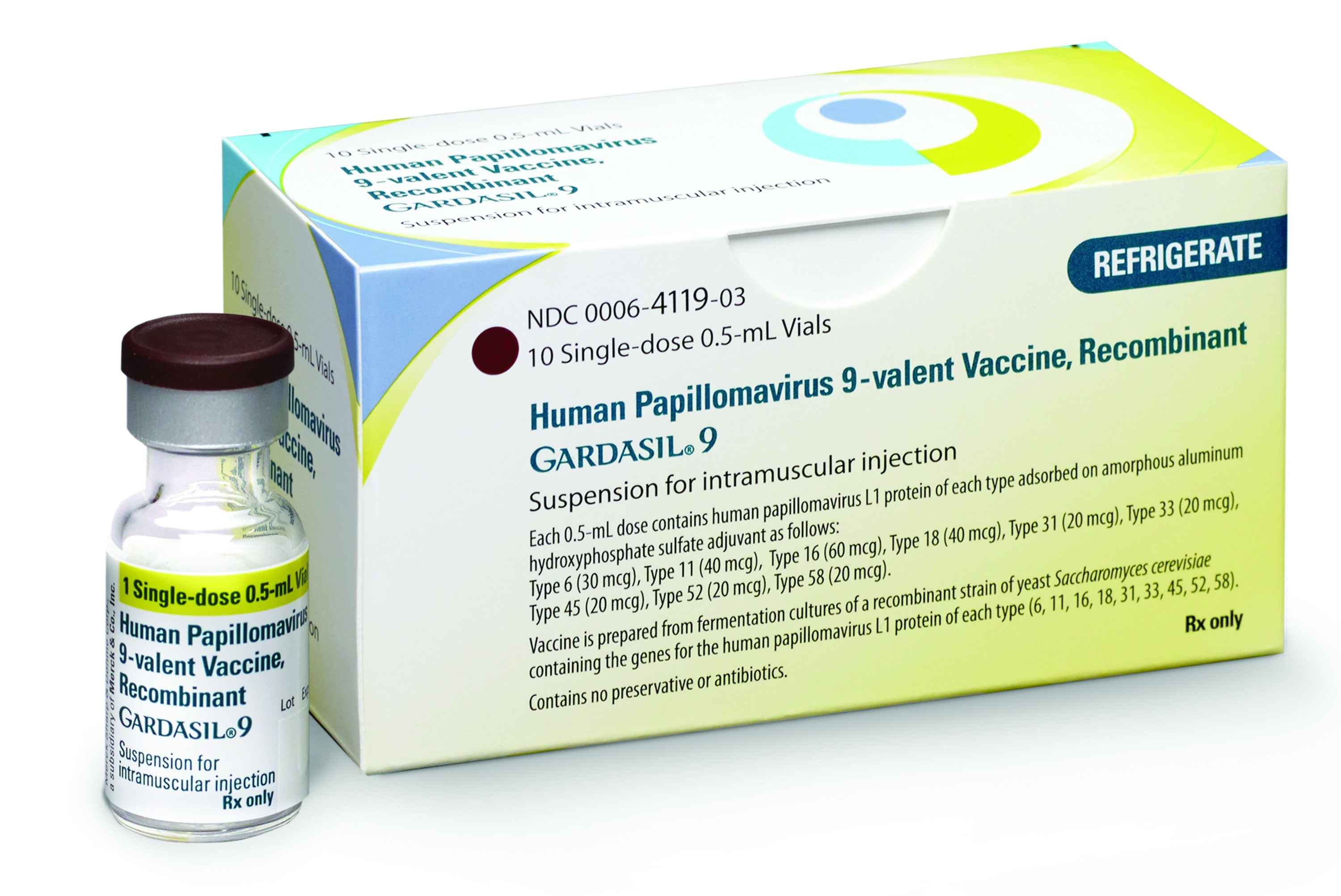
The U.S. Food and Drug Administration approved an expanded indication for Merck’s GARDASIL 9 for the prevention of oropharyngeal and other head and neck cancers caused by HPV Types 16, 18, 31, 33, 45, 52, and 58.
Pfizer and EMD Serono, the biopharmaceutical division of Merck KGaA, jointly announced that their Phase III JAVELIN Head and Neck 100 trial of Bavencio (avelumab) with chemoradiotherapy (CRT) for untreated locally advanced squamous cell carcinoma of the head and neck was unlikely to hit the primary endpoint.
FDA Grants Breakthrough Therapy Designation for Debiopharm’s Novel Chemo-Radio Sensitizer
Apoptosis Proteins, Breakthrough Therapy Designation, European Society for Medical Oncology (ESMO), FDA, FDA/Regulatory, Head & Neck Cancer, Locally Advanced Squamous Cell Carcinoma of the Head and Neck (LA-SCCHN), ProteinsDebiopharm announced that the U.S. Food and Drug Administration granted a Breakthrough Therapy Designation for Debio 1143, the most clinically advanced IAP antagonist, for the treatment of patients with confirmed diagnosis of previously untreated, unresectable locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN) in combination with current standard of care (CRT).
Top 200 Medicines Annual Report 2019: The king of medicines
Ankylosing Spondylitits, August 2019, Autoimmune Diseases, Biologics, Biosimilars, Blockbusters, Cervical Cancer, Classical Hodgkin Lymphoma, Crohn's Disease, Esophageal Cancer, Forecasts, Gastric Cancer, Head & Neck Cancer, Hepatocellular Carcinoma, Hidradenitis suppurativa, Issue Archives, Juvenile Idiopathic Arthritis, Market exclusivity, Melanoma, Merkel cell carcinoma, Metastatic Urothelial Carcinoma (mUC), Microsatellite instability-high (MSI-H) cancer, Monoclonal Antibodies, Non-Small Cell Lung Cancer (NSCLC), Plaque Psoriasis, Primary lediastinal large B-cell lymphoma, Product Launches, Psoriatic Arthritis, Renal Cell Carcinoma (RCC), Rheumatoid Arthritis, Therapeutics, Top 200 Medicines, Ulcerative Colitis, UveitisHumira’s dominance continues as the world’s top-selling prescription product as the biologic therapy is the first drug to exceed $20 billion in annual global sales.
Merck & Co. Inc.’s blockbuster cancer drug Keytruda won approval from the U.S. FDA to treat a type of head and neck cancer.
Galera Therapeutics Inc.announced that the clinical-stage biotechnology company secured $150 million in a joint, oversubscribed Series C financing and royalty purchase agreement.


 Getty Images
Getty Images