The U.S. Food and Drug Administration accepted for priority review the Biologics License Application submitted by Janssen Biotech Inc. for ciltacabtagene autoleucel (cilta-cel). The investigational B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T cell (CAR-T) therapy is intended for the treatment of patients with relapsed and/or refractory multiple myeloma (MM).
An experimental Bristol Myers Squibb drug from a new class of immunotherapy used in combination with the company’s big-selling cancer medicine Opdivo significantly extended the time it took for advanced melanoma to worsen compared with Opdivo alone, according to early data from a study released on May 19.
Jerusalem-based SpliSense, which focuses on cystic fibrosis and other genetic pulmonary diseases, closed on a $28.5 million Series B financing round. Participating in the round were OrbiMed, Israel Biotech Fund, Integra Holdings and the Cystic Fibrosis Foundation.
Researchers from Albert Einstein’s College of Medicine are excited with the recently published findings of an experimental drug that reversed key symptoms of Alzheimer’s disease in mice.
Mission Bio launched the company’s Pharma Assay Development (PAD) service, enabling its customers to obtain early access services based on the newest technologies as well as on existing products.
According to a research collaboration among UCLA investigators and Merck KGaA, berzosertib has shown promise for Covid-19 and is also being touted for the drug’s unusually broad effect in treating a range of cancers.
A readout from a Phase IIb/III trial showed intravenous RLF-100 (aviptadil) met the primary endpoint of improving survival and recovery at 60 days post-treatment for respiratory failure in critically ill patients with Covid-19, bolstering the case for sponsors NeuroRx and Relief Therapeutics to reapply for emergency use authorization (EUA).
U.S. FDA Approves Abecma for Relapsed or Refractory Multiple Myeloma
Approvals, B-cell maturation antigen (BCMA), Bristol Myers Squibb, CAR-T Therapy, Clinical Trials, Cytokine Release Syndrome (CRS), FDA/Regulatory, First-In-Class, Human Anti-CD38 Monoclonal Antibody, Immunomodulatory Agents, Proteasome Inhibitors, R&D, Relapse/Refractory Multiple Myeloma, TherapeuticsThe U.S. Food and Drug Administration approved Bristol Myers Squibb’s and bluebird bio’s Abecma (idecabtagene vicleucel) as the first anti-BCMA CAR T cell therapy for relapsed or refractory multiple myeloma.
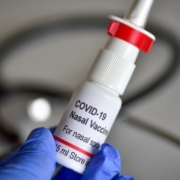
Meissa’s Nasal Covid-19 Vaccine Uses Codon Deoptimization for Stronger Responses
Business, Clinical Trials, Coronavirus Disease (COVID-19) Pandemic, Coronavirus Disease 2019 (COVID-19), COVID-19 immunity, Fast Track Designation, Immune Response, R&D, Respiratory Syncytial Virus (RSV) Infection, SARS-CoV-2 spike proteinMeissa Vaccines’ intranasal live attenuated chimeric virus-based vaccine for SARS-CoV-2 (and its variants), which was cleared for Phase I trials, employs codon deoptimization to evoke a stronger-than-usual immune response.
Roivant Sciences, the umbrella company for Vivek Ramaswamy’s series of ‘vant companies, acquired Silicon Therapeutics for $450 million in Roivant equity.