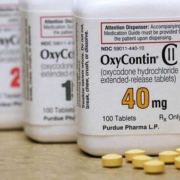
A federal judge on Feb. 1 extended a legal shield protecting the Sackler family owners of Purdue Pharma from lawsuits to Feb. 17, as they try to reach a deal with several states to settle sprawling litigation stemming from the U.S. opioid crisis.
The U.S. Food and Drug Administration approved Takeda Pharmaceutical Company Limited’s Vonvendi [von Willebrand factor (Recombinant)] for routine prophylaxis to reduce the frequency of bleeding episodes in patients with severe Type 3 von Willebrand disease (VWD) receiving on-demand therapy.
Members of the Sackler family who own Purdue Pharma LP are nearing an agreement to boost their more than $4 billion offer to resolve sprawling opioid litigation after negotiating with states that had objected to terms of the OxyContin maker’s bankruptcy reorganization, according to a court filing.
FDA Approves Genentech’s Vabysmo, the First Bispecific Antibody for the Eye, to Treat Two Leading Causes of Vision Loss
Approvals, Bispecific Antibody, Blood Vessels, Diabetic Macular Edema, FDA/Regulatory, Genentech, Ophthalmics, Roche, Therapeutics, Vascular Endothelial Growth Factor-A (VEGF-A) Protein, Vision Loss, Wet Age-Related Macular DegenerationThe U.S. Food and Drug Administration approved Genentech’s Vabysmo (faricimab-svoa) for the treatment of wet, or neovascular, age-related macular degeneration (AMD) and diabetic macular edema (DME).
Eli Lilly says FDA could deny expanded use of arthritis drug for eczema
AbbVie, Aopecia Areata, Arthritis, Blockbusters, Clinical Trials, Eczema, Eli Lilly, FDA, Hair Loss, Hospitalized COVID-19 Patients, Janus Kinase (JAK) Inhibitors, New Indications, Pfizer, Priority Review Status, R&D, Rivalries, TherapeuticsEli Lilly said on Jan. 28 the company expects the U.S. Food and Drug Administration to decline the approval of expanded use of the rheumatoid arthritis drug Olumiant as a treatment for adults with moderate-to-severe eczema.
Biogen is selling the company’s nearly 50 percent stake in South Korea-based Samsung Bioepis for $2.3 billion.
Regeneron Pharmaceuticals and the company’s partner Sanofi voluntarily withdrew their application with the U.S. drug regulator for the expanded use of the anti-cancer drug Libtayo in patients with advanced cervical cancer.
Merck & Co. Inc. and partner Ridgeback Biotherapeutics said on Jan. 28 six lab studies showed their experimental oral COVID-19 drug molnupiravir was active against the fast-spreading Omicron variant.
While the Centers for Medicare & Medicaid Services undergoes the comment period on its national coverage decision for Biogen’s controversial Alzheimer’s drug Aduhelm (aducanumab), Biogen and its partner company Eisai released additional details about the Phase IV post-marketing study of the drug.
Studies Forge On as Omicron Surges and More COVID-19 News
BNT162b2 (Pfizer and BioNTech), Clinical Trials, Coronavirus Disease (COVID-19) Pandemic, COVID-19 Studies, COVID-19 Therapeutics, COVID-19 Vaccines, Data, Hospitalized COVID-19 Patients, Inhaled Formulations, Janssen COVID-19 Vaccine (J&J), mRNA-1273/Moderna COVID-19 Vaccine (Moderna), New England Journal of Medicine, Omicron (B.1.1.529) (South Africa), R&D, Therapeutics, VentilatorsIn the last two years, the sheer volume of scientific research focused on COVID-19 has been astounding: thousands of clinical studies, dozens of vaccines and new compounds, and hundreds of approved drugs tested for efficacy. More studies are still being run, especially in light of the Omicron surge and the virus’ ability to evolve.