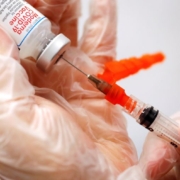
Pfizer Inc. said on Wednesday booster doses of the company’s COVID-19 vaccine can be administered along with its pneumonia vaccine and produced strong safety and immune responses in people aged 65 and above in a late-stage study.
India’s Bharat Biotech said on January 12 a booster shot of the company’s Covaxin COVID-19 vaccine administered six months after the last of two doses neutralizes both the Omicron and Delta variants of the coronavirus.
The U.S. Food and Drug Administration on January 11 amended the fact sheet for Johnson & Johnson’s COVID-19 vaccine to include a rare risk of immune thrombocytopenia, a bleeding disorder.
With record-shattering new cases of COVID-19 across the United States, the urgency for vaccinations is even greater than ever. In addition, Pfizer reported that an Omicron-specific vaccine will be available in March, should it be necessary.
BioNTech said the German COVID-19 vaccine maker developed a method to quickly determine whether a new virus variant is a cause for concern, collaborating with British artificial intelligence startup InstaDeep Ltd.
A U.S. federal judge in Texas denied attempts by the U.S. Food and Drug Administration to conceal data on Pfizer’s COVID-19 vaccine.
Two doses of the Pfizer Inc. and BioNTech SE COVID-19 vaccine are highly protective against a rare but often serious condition in children that causes organ inflammation weeks after COVID-19 infections, a U.S. Centers for Disease Control and Prevention report said on January 7.
The U.S. Food and Drug Administration on January 7 shortened the interval between the primary series of Moderna Inc.’s COVID-19 vaccine and a booster dose to five months, as it looks to bolster protection against the fast-spreading Omicron variant.
Exclusive: U.S. health agency may be unprepared to take over COVID vaccine program
"Operation Warp Speed" Initiative, BioNTech, BNT162b2 (Pfizer and BioNTech), Coronavirus Disease (COVID-19) Pandemic, COVID-19 Vaccinations, COVID-19 Vaccines, Department of Health and Human Services (HHS), Moderna, mRNA-1273/Moderna COVID-19 Vaccine (Moderna), Pfizer, Reuters, United StatesThe U.S. Department of Health and Human Services (HHS) appears unprepared to assume full responsibility for the nation’s Covid-19 vaccine program, including activities currently managed by the Pentagon, according to a draft government watchdog report reviewed by Reuters.
Johnson & Johnson said on January 6 that a real-world study showed the company’s single shot Covid-19 vaccine protects against breakthrough infections and hospitalizations for up to six months.