Efficiencies and adjacencies: Important considerations for effective clinical CRO partnerships
Efficiencies and adjacencies: Important considerations for effective clinical CRO partnerships
By Michael Towse & Frances Murphy
As a pharma or biotech company, few milestones are as exciting as the completion of pre-clinical testing with positive results. With entry to the clinic imminent, this is often the moment you first seek to engage a clinical contract research organization (CRO). Experience suggests that beginning the process sooner is often better, but regardless of the timing, this is the opportunity to ask yourself several important questions, including:
- What should we think about before approaching a CRO?
- Which CRO(s) should we approach, and how?
- How do we evaluate and compare each CRO’s offerings and capabilities?
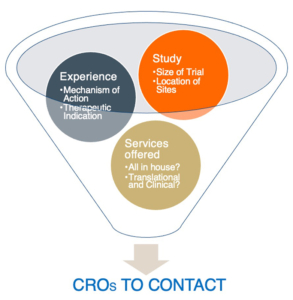
As with many aspects of clinical research, meticulous preparation can optimize and expedite the CRO selection process. Whereas most CROs offer similar services — ranging from project management and monitoring to biostatistics and data management — each has points of creative differentiation that should inform the selection process. It is also crucial to determine whether all your service needs are to be managed by one CRO or by several, as well as if those services are to include lab support and kitting in addition to clinical tasks. Other important considerations are your planned study size and location, and whether the CRO has the necessary experience in the therapeutic area, mechanism of action, or drug delivery vehicle (see Figure 1).
Answering these questions should generate a preliminary list of potential CROs. However, before initiating contact, it is important to determine the ultimate number of CROs to include in your outreach. In general, it makes sense to solicit proposals from three to five CROs, and to conduct follow-up bid defense meetings (BDMs) with two or three, based on the quality of the responses. Keep in mind that it takes considerable time and manpower for a CRO to produce a custom proposal, which may amount to 30–50 pages. Just as importantly, reviewing and evaluating each proposal can be very time- and resource-intensive for you. Engaging a CRO is the start of a long-term relationship, and it is crucial to get off on the right foot.
Once you’ve built your preliminary list, your professional networks can be a rich source of information on potential candidates, as can your own personal networks. If the study’s lead investigator is already on board, or if you maintain relationships with key opinion leaders, they may have some insights. Whatever your preferred shortlisting method, it is also advisable to reach out to CROs for an initial conversation. These early conversations can also be useful in discerning which CROs themselves are most interested in the study you’ll be conducting.
Submitting the RFP
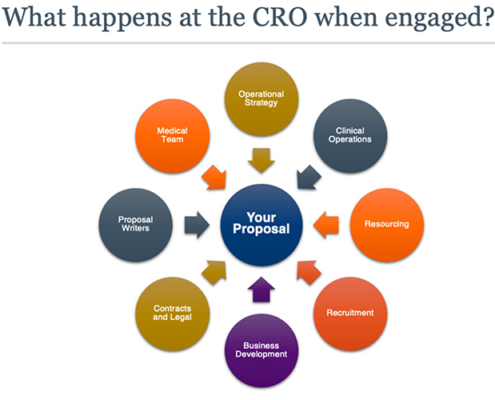
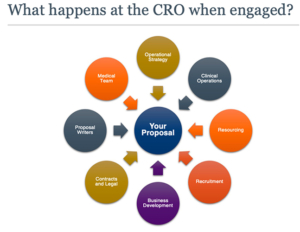
After some exploratory conversations, and perhaps a capabilities demonstration, it is time to issue a request for proposal (RFP) to your shortlisted CROs. Normally, once received, a multi-disciplinary group of each CRO’s subject matter experts (SMEs) — an example is depicted in Figure 2 — triages the RFP to determine whether the company can fulfill the specified deliverables. The SME team then assembles the proposal, which typically encompasses landscape and feasibility assessments, site outreach, medical insights, strategy, budget, and team profiles. Developing the proposal is a deeply collaborative effort, which is why a 10-working day turnaround time is standard, with perhaps an extension over a particularly busy part of the year.
The proposal will also include assumptions, particularly if any aspect of the RFP is unclear. Therefore, the more information you share in the RFP, the better the proposal output – and the more worthy of your review time. CROs will often provide an RFP Checklist to help ensure the capture of the right information. Most CROs understand that project funding may depend on attainment of key milestones, such as regulatory submissions and enrollment of the first patient. With information about those targets, the CRO can devise a plan to help achieve them.
Throughout the process you will have an obligation to inform the CRO of any change in project parameters which might necessitate adjustments to the proposal. The RFP process is also a good time to gauge the chemistry between your team and the CRO, as you will plan to be partners for the next few years, through times both rewarding and challenging.
BDM, award and budget
As noted, you should ideally receive several proposals, each informed by the same parameters to enable a like-for-like comparison. However, that may limit CRO creativity. When granted more flexibility, CROs often come back with ideas and opinions on trial design, location, and other factors you may not have yet considered.
After review and evaluation, the top two or three CROs should be invited to present a Bid Defense, and as a key facet of that invitation, your teams should clearly communicate the topics to be addressed during the BDM. Otherwise, the CRO will make assumptions about clinical trial priorities based on experience. The importance of respecting the CRO’s time cannot be overstated, given the Bid Defense team’s extensive preparation and the number of contributors to the proposal. Since the height of the pandemic, BDMs have started to return to an in-person format, although hybrid formats (in-person and virtual) are often the favorite. They typically last about three hours, with much of that time devoted to medical and strategy discussions, and there may not be enough time to cover every topic. That reality underscores the need for pragmatism and realistic expectations. Set these upfront and ensure you are able to discuss the critical aspects leading to study success.
Ideally, there will be follow-up and discussion post-BDM. In our experience, it’s important that these interactions are dialogues, not monologues, and that they flow from informed questions asked during the meeting and from constructive feedback in its aftermath. In this way, you will foster an effective process which lays the groundwork for an excellent partnership and a successful clinical trial experience.
The industry norm is for the sponsor to make its selection within three weeks of the BDM. This is especially important if you expect to retain the team that presented at the meeting. The CRO’s teams may be in contention for several clinical trial projects, a reality that highlights the need for speed between BDM and award notification.
Finally, a word on budget. Notably, the first budget submitted by the CRO will be a draft and should not be the deciding factor in the selection process. If there are strict budgetary constraints, the CRO will likely be prepared to be flexible to work within those parameters, and to adjust the budget where possible. Competing budget quotes should be compared and understood, but the final decision should rest on the overall quality of the proposal. Once that selection is made, the budget negotiations can, and often do, truly begin.
Summary
In short, the selection process is a multifaceted endeavor. At its best, it is the result of considerable time, thought, effort, and resources from both sponsors and the prospective CROs.
As such, the decision of whom to choose should not hinge on a single component of the CRO’s proposal, whether it’s the budget, the site plan, the investigator roster, or the overall project timeline. Rather, your decision should be based on the totality of the proposal, including the CRO’s expertise, flexibility, and creativity. When you consider those factors as key criteria, it can lead not only to a successful study, but to a long-term, value-added partnership that will aid in your quest for therapeutic solutions. With a capable CRO in your corner, you can pursue your mission of addressing unmet patient needs. That makes it especially important to choose your CRO partner wisely.