Q&A with OptumRx Senior Director Bill Dreitlein
Med Ad News talked to Bill Dreitlein – Senior Director, Pipeline & Drug Surveillance at OptumRx – about some of the most promising new molecular entities in the industry pipeline and challenges the FDA has faced during the COVID-19 pandemic.
By Andrew Humphreys • [email protected]
Med Ad News: What are the potential blockbuster products to watch from the class of 2020 U.S. drug approvals?
Bill Dreitlein: Risdiplam for SMA. As an oral treatment for SMA, Risdiplam is compelling and could reach blockbuster status in 2022, according to some financial forecasts.
Remdesivir for COVID-19. Remdesivir is already a blockbuster earning ~$2B in 2020 so it bears watching what will happen as its commercial performance may track with the COVID-19 epidemic.
Med Ad News: What are some of the most promising NMEs in the pipeline expected to win FDA approval in 2021 or later?
Bill Dreitlein: If approved, Roxadustat would be the first oral treatment option and novel mechanism of action for CKD-related anemia, which will be competing with intravenous (IV)/subcutaneous (SC) administered erythropoiesis-stimulating agents (ESAs). There may be alternative benefits around safety (e.g., reduced need for iron supplementation). For patients self-administering SC administered ESAs, an oral alternative may be beneficial as a more convenient route of administration and may lower the barrier to treatment.
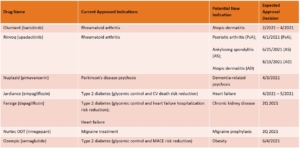
A closely watched drug category in 2021 will be atopic dermatitis drugs, including the JAK inhibitors abrocitinib and a topical formulation of ruxolitinib (currently only available as an oral product [Jakafi]). These will be competing with currently available JAK inhibitors that are also pursuing new indications for atopic dermatitis – Rinvoq and Olumiant.
Med Ad News: Despite the pandemic, the amount of novel drug approvals in the U.S. during 2020 increased compared to 2019 and represented the second-highest total in 10 years. What is the outlook for 2021, and what challenges may the FDA face in the continuing COVID-19 landscape?
Bill Dreitlein: Overall, while trial schedules have slowed, they have not stopped, and we see 2021 following a similar pattern. Regarding new drug launches, we have previously noted instances where new drugs have received approvals, but manufacturers decided to delay launch due to pandemic related concerns. However, drug makers have adapted, and we are now seeing “virtual” drug launches that minimize person-to-person contact by substituting virtual doctor’s office visits and advisory boards, instead of in-person.
As the new year begins, it does not appear that the pandemic has slowed the FDA’s approval process. The graph in our OptumRx Q4 Drug Pipeline report combines approvals in past years with 2020 year-to-date values, and shows that the number of new drugs this year could equal or even exceed prior years.
Med Ad News: Among products already available in the U.S. market, what are some of the significant new indications/delivery systems in late-stage development that will help drive blockbuster growth?