The FDA weighs in on market research and drug developers are listening – especially to the underserved
The FDA weighs in on market research and drug developers are listening – especially to the underserved
By Fabio Gratton
A new era of FDA guidance
The foundational value of research for marketing goes back to the pioneering work of Daniel Starch in the early 1920s, when data collection, analysis, and reporting were systematized for the first time. Since then companies across most verticals have spent billions to gain an advantage through an improved understanding of their competitors and consumers. Within our own healthcare industry, market research designed to help brands better empower patients and professionals to make the best treatment choices has similarly become table stakes.
Thanks to the meteoric rise of digital health, the role of data and analytics has also become central across the drug development life cycle, impacting every touch point from pipeline research and development to clinical trial design and implementation. A critical area that has fallen short, however, is applying a commensurate level of enthusiasm and expense for market research in the early phase as that afforded to downstream commercialization efforts. Compounding this challenge is the continued marginalization of disenfranchised communities.
Important studies shared from the Center for Information and Study on Clinical Research Participation (CISCRP) reveal significant ongoing disparities in the representation of underserved populations, including disproportionately lower participation in clinical trials from African-American, Asian, and Hispanic subpopulations. The inclusivity problem throughout drug development is so urgent as to implore the FDA to formally recommend the broadening of eligibility criteria to better reflect the demographics of patients needing and using treatments.
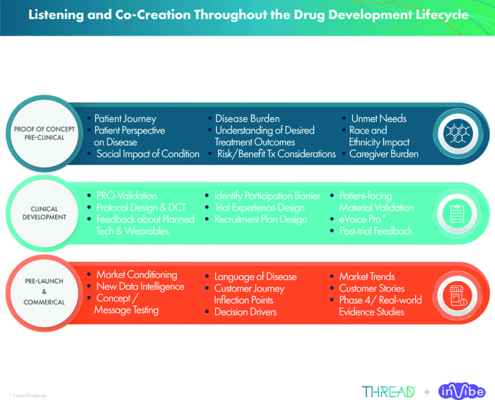
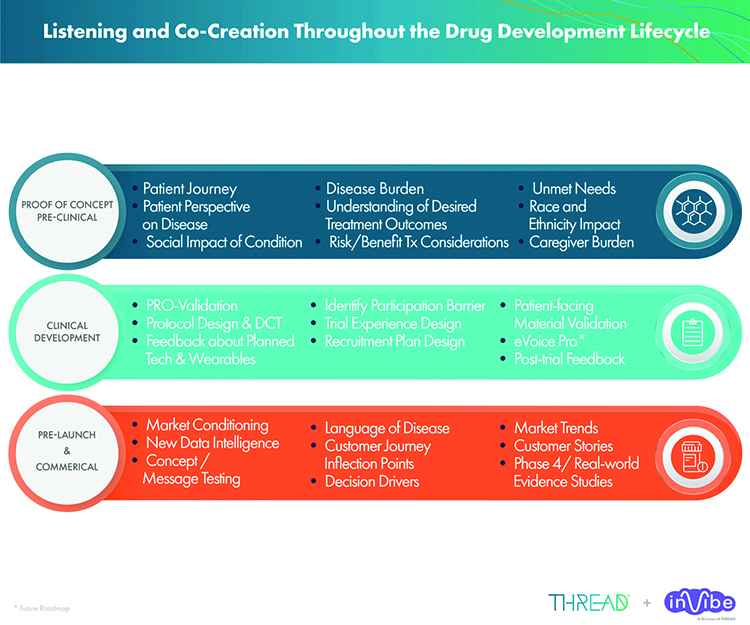
The agency has also recognized the critical role market research plays to improve clinician understanding of patient needs and expectations throughout the early phase of the lifecycle, and has recently developed a series of four patient-focused drug development (PFDD) guidance documents. An extension of Congress’ 21st Century Cares Act to include patient experience data in support of regulatory decision making and medical product development, the goal of this new guidance is to assist in the accurate and comprehensive collection and submission of data.
The second installment was issued in February of this year, and entitled “Methods to Identify What Is Important to Patients.” The document provides an overview of qualitative and quantitative market research techniques, highlighting the strengths and weaknesses of each approach. Written to help inform and optimize clinical trial design, the acquisition of patient experience data, and clinical outcomes assessments, the FDA guidance gets to the heart of how to optimally reveal patient sentiment around the burden of disease and its treatments.
Removing burdens and creating opportunities
Pharma marketers must typically choose between two distinct options for market research as outlined in the new guidance: quantitative techniques that involve broadly distributed and quickly processed surveys, or qualitative ones that consist of in-depth and time-consuming interviews and focus groups. Both methods have unfortunately fallen short for historically disenfranchised communities, where the anonymity of technology barriers of the former, and the inconvenience and biases of the latter, are all compounded by language hurdles and systemic biases.
Sensitive to the plight of those marginalized by the healthcare system for these and other reasons, the new guidance places special emphasis on overcoming what it defines as the “respondent burden.” Special focus is given to test subjects with distinct physical, sensory, educational, and communication needs and styles; sensitivities to diverse abilities and cultural backgrounds is emphasized; and the impediments of travel, time off from work, and other logistical factors are called out to ensure equal representation and seamless self-reporting.
To overcome these and other hurdles, the FDA recommends a full spectrum of approaches ranging from specific data collection tactics to broader co-creation strategies. Simplified and efficient survey designs and layouts are a great starting point, featuring health literate language, good translations into multiple languages, straightforward and non-repetitive questions, and the importance of pretesting content with diverse patient audiences. Sensitivities to diverse cultural nuances, eclectic communication styles, and utilization of a shared lexicon are also key.
Significantly, the FDA also places strong emphasis on the active and continuous co-creation of content. Market researchers and the companies they represent are advised to actively partner with disease experts, statisticians, psychometricians, and the patients and clinicians themselves as a core component of the discovery and content development processes. The goal is to eliminate as much of the respondent burden as possible by better understanding patients and their needs, then applying those insights to remove friction at every communication touch point.
If successful, core clinical trial components can be optimized to ultimately improve recruitment and retention rates, especially for the disenfranchised. The co-creation of educational materials, recruiting and compliance strategies, and even clinical trial design parameters can help make patient-focused content and experiences more impactful, convenient and utilized. Despite these obvious benefits, many of the foundational approaches to data collection, analysis and reporting remain burdensome for traditional qual and quant market research. What’s next?
A phone in every pocket, a voice in every study
Part of the challenge is that clinicians and even downstream marketers consider research an enormous hassle. Qualified patients are difficult to find and engage, and as the FDA guidance shows, many techniques remain cumbersome and time consuming. For clinical trials, protocols have often developed to solve some problems only to create new ones, including participation in the trials themselves, typically designed by experts with little understanding of their patients’ needs and expectations. So how can friction best be removed, and patients actually listened to?
The evolution and now dominion of mobile communications technology offers a hint. A vast majority of all Internet traffic takes place on mobile devices, our smartphones accessed on average hundreds of times per day. Decades ago pundits predicted flying cars and bases on the Moon, few anticipating that the most transformative innovation would prove to be a miniature computer in each of our pockets, enabling instantaneous and global connectivity to people, information, entertainment, and endless products and services, destroying whole industries.
Everyone has a phone, and everyone loves to talk. Irrespective of economic status and cultural preference, circumventing racial and cultural bias and even discrimination, Web-connected phones have given everyone a voice. The advent of social media has amplified these voices, while the ascendance of digital on-demand services such as Amazon, Uber and Airbnb have brought unprecedented immediacy, personalization convenience, and consumer power to billions of people worldwide. The overall result: a democratization of society like never before.
Virtually overnight, the age-old boundaries between governments and citizens, celebrities and fans, brands and consumers dissolved. The “Big Idea” for market research is that the boundary between clinical trials and patients should also dissolve thanks to innovative approaches to data collection and analysis. Why rely exclusively on tedious, anonymous, and impersonal surveys or uncomfortable and potentially biased interviews and focus groups when patients – who already have phones and love to talk – can’t wait to express themselves if only given the chance?
So let’s give patients that chance, especially those from diverse backgrounds and communities. The ubiquity of telephony creates equal opportunity, flattens the playing field, and destroys systemic barriers to entry. Emboldening minorities to candidly speak for themselves is especially necessary for subpopulations with the multiple burdens of neglect, inequality, language, and limited tech – all made worse by the global pandemic. Ostensibly designed to heal, the system makes diverse patients sick with confusion and feelings of inadequacy. They need an out.
The dawn of inclusive voice-response and crowdsourced research
Attaining the aspirational goals of a truly patient-focused drug development process demands inclusivity. PFDD de facto demands positioning patients from diverse racial and ethnic backgrounds to fully inform the therapeutic context and counsel lifesciences companies on the best ways to recruit and retain them for clinical trials. Mountains of money and herculean efforts have already been made, but the missing link has been empowering the patients.
By asking patients specific, open-ended questions about clinical trial assets, design parameters, and other stimuli, then recording their candid responses directly from their own phones, clinicians can finally hear and integrate insights from thousands of voices once ignored or silenced. Clinicians can finally hear what their patients think about trial designs and assets, their responses analyzed with a combination of human linguistic expertise and machine learning technology. Meaningful insights can then be translated into actionable recommendations.
Major pharma companies have already tapped into this innovative caliber of voice-response market research for drug development. Actionable insights cover the gamut from the need to create more health literate and culturally sensitive educational materials for trial recruitment, to the favored use of decentralized clinical trials that are more convenient for disadvantaged patients having difficulties traveling to centers, or allocating the necessary time away from their demanding jobs. For the first time, patients are able to co-create ideal content and experiences.
Yet another “Big Idea” for integrating diverse patient voices directly into clinical processes is through the crowdsourcing of social media health influencers. Since patients are far more likely to trust other patients who share their concerns than pharma companies that design and implement trials, the idea is to find and empower already active and cherished influencers to pass along trial information and encourage participation. Advocacy groups and advisory boards can help refine and guide the relationships between brands, patients and influencers.
For the first time, patients are empowering each other to help drive the development of vital treatments – while clinicians are finally able to listen to and integrate their personal feedback every step of the way. More than 100,000 patients and nearly 500 recruitment campaigns have been positively impacted by this novel approach to clinical trial recruitment, while thousands more patients and professionals have recorded terabytes of voice data, ultimately converted to insights and recommendations transforming trials and treatments.
The democratization of healthcare market research
People naturally love to talk, and lifesciences companies have for decades been eager to hear everything they want to say. The missing link has been figuring out how to find and connect patients from all walks of life with busy clinicians obsessed with every aspect of the drug development process – except the critically vital needs and expectations of its end user, their patients. By making the entire market research process quick, convenient and effective, patients regardless of background are finally able to speak, and pharma is eager to listen.
The essence of success is paradoxically found in pharma companies relinquishing control. Rather than build trials patients are forced to adapt to, and recruit for trials exclusively through CROs, drug developers are learning how to trust and empower the patients themselves to learn about, understand, and ultimately guide each other and the industry throughout the clinical trial design and implementation process. The bottom line is that trust can only happen both ways, and when the means of communication is scalable, transparent and equalitarian.
The digital revolution has revolutionized how we communicate, and transformed our lives. Accustomed to immediate, personalized and convenient interactions between people and products, we’ve embraced on-demand services of every type, and tacitly expect nothing less from the most important industry of all, healthcare. Once marginalized and disparaged populations are excluded no more, and make their voices heard and demands met. Never before has pharma had such an opportunity to engage, nor have expectations been higher.
Along the way, clinicians once skeptical of the time, expense, and utility of integrating diverse patient voices throughout the drug development process have become better listeners. Innovative market research strategies and tech help connect stakeholders like never before, bringing thrilling and unprecedented levels of patient-centricity to what was once the exclusive domain of academics and corporations. The results are already in: increased recruitment and retention rates, and improved outcomes from better chosen and evaluated drugs.
My wife and I are both immigrants, our children multicultural, and we enthusiastically embrace the vision of an increasingly diverse, inclusive country. Mobile technology and social media have triggered their fair share of societal challenges, but I remain a Pollyanna about the potential of technology to make the world a better place. Central is the hope that the tech is merely a tool, driven by an intent to use such incredible power for good. By enabling millions who have been silenced to speak, we’re also encouraging noisy companies to listen, improving health and wellness for all.
Digital health entrepreneur Fabio Gratton is co-founder and CEO of inVibe and CureClick, divisions of THREAD Research.