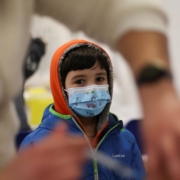
The Massachusetts Department of Public Health on May 17 said it had confirmed a single case of monkeypox virus infection in a man who had recently traveled to Canada.
COVID Americas cases up, North American cases up for 5th week – PAHO
Canada, Central America, Coronavirus Cases, Coronavirus Disease (COVID-19) Pandemic, COVID-19 cases, COVID-19 Infections, Hospitalizations, Hospitalized COVID-19 Patients, New Mexico, North America, Pan-American Health Organization (PAHO), Pandemics, Symptomatic COVID-19 Infections, Therapeutics, United StatesCOVID-19 cases in the Americas increased by 12.7 percent last week from the prior week, the Pan American Health Organization (PAHO) said on May 4, as infections continued to rise in Central and North America.
Pfizer said on April 22 the company was voluntarily recalling five batches of Accupril blood pressure tablets after finding elevated levels of a potential cancer-causing agent in the medicine.
COVID cases down in the Americas even as North America faces increase – PAHO
BNT162b2 (Pfizer and BioNTech), Canada, Coronavirus Disease (COVID-19) Pandemic, Coronavirus Vaccines, COVID-19 cases, COVID-19 Deaths, COVID-19 Vaccines, Covid-19 Variants, Hospitalized COVID-19 Patients, Janssen COVID-19 Vaccine (J&J), mRNA-1273/Moderna COVID-19 Vaccine (Moderna), North America, Omicron BA.2, Pan-American Health Organization (PAHO), Pandemics, TherapeuticsCOVID-19 cases and deaths are declining in the Americas, the Pan American Health Organization (PAHO) said on April 20, with infections last week having dropped 2.3 percent and deaths falling 15.2 percent from the prior week. The broad trend comes even as cases have scaled up in North America with an 11.2 percent increase last week, the organization said.
Medicago’s vaccine on February 24 became the world’s first plant-based shot approved against COVID-19 after Health Canada cleared Covifenz for use in adults.
The U.S. Department of Homeland Security (DHS) announced it is requiring that non-U.S. essential workers such as truck drivers and nurses who are crossing land borders be fully vaccinated against COVID-19, effective January 22.
COVID-19 infections are reaching new peaks in the Americas with 7.2 million new cases and more than 15,000 COVID-related deaths in the last week, the Pan American Health Organization (PAHO) said on January 19.
Scarborough Health Network Foundation launched a historic new campaign, “Love, Scarborough,” to support SHN’s goal of raising $100 million in funds to help close the healthcare gap. The integrated campaign, created by Ogilvy Toronto, is being featured across TV, cinema, print, radio and online.
Covid-19 deaths and infections declined across the Americas for an 8th consecutive week, the Pan American Health Organization (PAHO) said on Nov. 3, warning that a very high percentage of hospitalized cases now are unvaccinated people.
Toronto-based charity The Shoebox Project for Women is partnering with Klick Health (its title sponsor) to launch a four-story art installation by Canadian artist Daniel Mazzone to put a spotlight on local women experiencing homelessness.




 Reuters
Reuters